Evaluation of the global association between cholesterol-associated polymorphisms and Alzheimer's disease suggests a role for rs3846662 and HMGCR splicing in disease risk
- PMID: 21867541
- PMCID: PMC3180274
- DOI: 10.1186/1750-1326-6-62
Evaluation of the global association between cholesterol-associated polymorphisms and Alzheimer's disease suggests a role for rs3846662 and HMGCR splicing in disease risk
Abstract
Background: Recent genome-wide association studies (GWAS) have identified single nucleotide polymorphisms (SNP)s that are essentially unequivocally associated with peripheral cholesterol. Since the alleles of the APOE gene, which modulate peripheral cholesterol metabolism, and midlife plasma cholesterol are both associated with Alzheimer's disease (AD) risk, we have evaluated the hypothesis that SNPs associated with plasma cholesterol are also associated with AD.
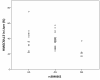
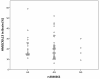
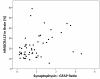
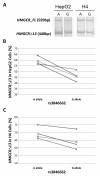
Results: Seventeen non-APOE SNPs reproducibly associated with cholesterol per GWAS were tested for association with AD in ~2,000 AD and ~4,000 non-AD subjects. As a group, these SNPs are associated with AD. Two SNPs in particular, rs3846662 and rs1532085, are associated with AD risk and age-of-onset. Additionally, rs3846662 was associated with HMGCR exon 13 splicing in human liver but not brain, possibly obscured by CNS cell-type heterogeneity. However, rs3846662 was associated with HMGCR exon 13 splicing in liver- and brain-derived cell lines.
Conclusions: Cholesterol-associated SNPs outside of APOE confer a global risk for AD. Rs3846662 and rs1532085 are associated with both AD risk and age-of-onset. Rs3846662 is associated with HMGCR exon 13 inclusion. Since rs3846662 affects AD risk and age-of-onset as well as statin responsiveness, this SNP may confound clinical trials evaluating the protective effects of statins on AD.
Figures


References
-
- Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, Posner SF, Viitanen M, Winblad B, Ahlbom A. Heritability for Alzheimer's disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52:M117–125. - PubMed
-
- Davidson Y, Gibbons L, Pritchard A, Hardicre J, Wren J, Stopford C, Julien C, Thompson J, Payton A, Pickering-Brown SM. et al. Apolipoprotein E epsilon4 allele frequency and age at onset of Alzheimer's disease. Dementia and geriatric cognitive disorders. 2007;23:60–66. doi: 10.1159/000097038. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

