Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus
- PMID: 21867881
- PMCID: PMC3272880
- DOI: 10.1016/j.neuron.2011.06.019
Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus
Abstract
Our understanding of mechanisms that regulate the differentiation of specific classes of synapses is limited. Here, we investigate the formation of synapses between hippocampal dentate gyrus (DG) neurons and their target CA3 neurons and find that DG neurons preferentially form synapses with CA3 rather than DG or CA1 neurons in culture, suggesting that specific interactions between DG and CA3 neurons drive synapse formation. Cadherin-9 is expressed selectively in DG and CA3 neurons, and downregulation of cadherin-9 in CA3 neurons leads to a selective decrease in the number and size of DG synapses onto CA3 neurons. In addition, loss of cadherin-9 from DG or CA3 neurons in vivo leads to striking defects in the formation and differentiation of the DG-CA3 mossy fiber synapse. These observations indicate that cadherin-9 bidirectionally regulates DG-CA3 synapse development and highlight the critical role of differentially expressed molecular cues in establishing specific connections in the mammalian brain.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
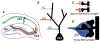




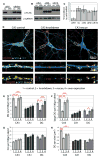


Comment in
-
Cadherins as matchmakers.Neuron. 2011 Aug 25;71(4):566-8. doi: 10.1016/j.neuron.2011.08.005. Neuron. 2011. PMID: 21867873
References
-
- Ackley BD, Jin Y. Genetic analysis of synaptic target recognition and assembly. Trends Neurosci. 2004;27:540–547. - PubMed
-
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. - PubMed
-
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. - PubMed
-
- Arikkath J. Regulation of dendrite and spine morphogenesis and plasticity by catenins. Mol Neurobiol. 2009;40:46–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

