CD8α(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites
- PMID: 21867928
- PMCID: PMC3171793
- DOI: 10.1016/j.immuni.2011.08.008
CD8α(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites
Abstract
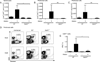
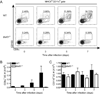
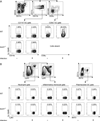
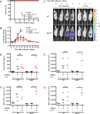
CD8α(+) dendritic cells (DCs) are important in vivo for cross-presentation of antigens derived from intracellular pathogens and tumors. Additionally, secretion of interleukin-12 (IL-12) by CD8α(+) DCs suggests a role for these cells in response to Toxoplasma gondii antigens, although it remains unclear whether these cells are required for protection against T. gondii infection. Toward this goal, we examined T. gondii infection of Batf3(-/-) mice, which selectively lack only lymphoid-resident CD8α(+) DCs and related peripheral CD103(+) DCs. Batf3(-/-) mice were extremely susceptible to T. gondii infection, with decreased production of IL-12 and interferon-γ. IL-12 administration restored resistance in Batf3(-/-) mice, and mice in which IL-12 production was ablated only from CD8α(+) DCs failed to control infection. These results reveal that the function of CD8α(+) DCs extends beyond a role in cross-presentation and includes a critical role for activation of innate immunity through IL-12 production during T. gondii infection.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures

Comment in
-
Intracellular pathogens and CD8(+) dendritic cells: dangerous liaisons.Immunity. 2011 Aug 26;35(2):153-5. doi: 10.1016/j.immuni.2011.08.003. Immunity. 2011. PMID: 21867923
References
-
- Aliberti J, Schulz O, Pennington DJ, Tsujimura H, Sousa Reis E, Ozato K, Sher A. Essential role for ICSBP in the in vivo development of murine CD8alpha + dendritic cells. Blood. 2003;101:305–310. - PubMed
-
- Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–4521. - PubMed
-
- Bliss SK, Zhang Y, Denkers EY. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-gamma-independent IL-12. J Immunol. 1999;163:2081–2088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

