Synthesis and biological evaluation of a peptide-paclitaxel conjugate which targets the integrin αvβ₆
- PMID: 21868241
- PMCID: PMC3195361
- DOI: 10.1016/j.bmc.2011.07.046
Synthesis and biological evaluation of a peptide-paclitaxel conjugate which targets the integrin αvβ₆
Abstract
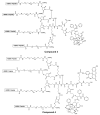
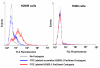
The integrin α(v)β(6) is an emergent biomarker for non-small cell lung cancer (NSCLC) as well as other carcinomas. We previously developed a tetrameric peptide, referred to as H2009.1, which binds α(v)β(6) and displays minimal affinity for other RGD-binding integrins. Here we report the use of this peptide to actively deliver paclitaxel to α(v)β(6)-positive cells. We synthesized a water soluble paclitaxel-H2009.1 peptide conjugate in which the 2'-position of paclitaxel is attached to the tetrameric peptide via an ester linkage. The conjugate maintains its specificity for α(v)β(6)-expressing NSCLC cells, resulting in selective cytotoxicity. Treatment of α(v)β(6)-positive cells with the conjugate results in cell cycle arrest followed by induction of apoptosis in the same manner as free paclitaxel. However, initiation of apoptosis and the resultant cell death is delayed compared to free drug. The conjugate demonstrates anti-tumor activity in a H2009 xenograft model of NSCLC with efficacy comparable to treatment with free paclitaxel.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures





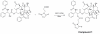

Similar articles
-
Synthesis and biological evaluation of dimeric RGD peptide-paclitaxel conjugate as a model for integrin-targeted drug delivery.J Med Chem. 2005 Feb 24;48(4):1098-106. doi: 10.1021/jm049165z. J Med Chem. 2005. PMID: 15715477
-
Selective apoptosis-inducing activity of synthetic hydrocarbon-stapled SOS1 helix with d-amino acids in H358 cancer cells expressing KRASG12C.Eur J Med Chem. 2020 Jan 1;185:111844. doi: 10.1016/j.ejmech.2019.111844. Epub 2019 Nov 2. Eur J Med Chem. 2020. PMID: 31706640
-
Diosmetin induces apoptosis and enhances the chemotherapeutic efficacy of paclitaxel in non-small cell lung cancer cells via Nrf2 inhibition.Br J Pharmacol. 2019 Jun;176(12):2079-2094. doi: 10.1111/bph.14652. Epub 2019 May 11. Br J Pharmacol. 2019. Retraction in: Br J Pharmacol. 2024 Apr;181(8):1342. doi: 10.1111/bph.16346. PMID: 30825187 Free PMC article. Retracted.
-
Synthesis and anti-cancer evaluation of folic acid-peptide- paclitaxel conjugates for addressing drug resistance.Eur J Med Chem. 2019 Jun 1;171:104-115. doi: 10.1016/j.ejmech.2019.03.031. Epub 2019 Mar 18. Eur J Med Chem. 2019. PMID: 30913525
-
Synthesis and biological evaluation of RGD peptidomimetic-paclitaxel conjugates bearing lysosomally cleavable linkers.Chemistry. 2015 Apr 27;21(18):6921-9. doi: 10.1002/chem.201500158. Epub 2015 Mar 17. Chemistry. 2015. PMID: 25784522
Cited by
-
Delivery of Theranostic Nanoparticles to Various Cancers by Means of Integrin-Binding Peptides.Int J Mol Sci. 2022 Nov 8;23(22):13735. doi: 10.3390/ijms232213735. Int J Mol Sci. 2022. PMID: 36430214 Free PMC article. Review.
-
A liposomal drug platform overrides peptide ligand targeting to a cancer biomarker, irrespective of ligand affinity or density.PLoS One. 2013 Aug 23;8(8):e72938. doi: 10.1371/journal.pone.0072938. eCollection 2013. PLoS One. 2013. PMID: 24009717 Free PMC article.
-
Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy.Pharmaceutics. 2023 Aug 29;15(9):2233. doi: 10.3390/pharmaceutics15092233. Pharmaceutics. 2023. PMID: 37765202 Free PMC article. Review.
-
Site-Specific Antibody Functionalization Using Tetrazine-Styrene Cycloaddition.Bioconjug Chem. 2018 May 16;29(5):1605-1613. doi: 10.1021/acs.bioconjchem.8b00114. Epub 2018 May 3. Bioconjug Chem. 2018. PMID: 29694034 Free PMC article.
-
Molecular imaging of integrin αvβ6 expression in living subjects.Am J Nucl Med Mol Imaging. 2014 Jun 7;4(4):333-45. eCollection 2014. Am J Nucl Med Mol Imaging. 2014. PMID: 24982819 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Xu J, Ward E. CA Cancer J Clin. 2010;60:277. - PubMed
-
- Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A, Hortobagyi GN, Ellerhorst JA. Clin Cancer Res. 2002;8:1038. - PubMed
-
- Henderson IC, Bhatia V. Expert Rev Anticancer Ther. 2007;7:919. (25) - PubMed
-
- Safavy A. Curr Drug Del. 2008;5:42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

