Membrane fluidity profiles as deduced by saturation-recovery EPR measurements of spin-lattice relaxation times of spin labels
- PMID: 21868272
- PMCID: PMC3214655
- DOI: 10.1016/j.jmr.2011.07.022
Membrane fluidity profiles as deduced by saturation-recovery EPR measurements of spin-lattice relaxation times of spin labels
Abstract
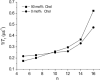
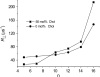
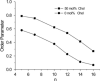
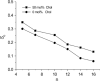
There are no easily obtainable EPR spectral parameters for lipid spin labels that describe profiles of membrane fluidity. The order parameter, which is most often used as a measure of membrane fluidity, describes the amplitude of wobbling motion of alkyl chains relative to the membrane normal and does not contain explicitly time or velocity. Thus, this parameter can be considered as nondynamic. The spin-lattice relaxation rate (T(1)(-1)) obtained from saturation-recovery EPR measurements of lipid spin labels in deoxygenated samples depends primarily on the rotational correlation time of the nitroxide moiety within the lipid bilayer. Thus, T(1)(-1) can be used as a convenient quantitative measure of membrane fluidity that reflects local membrane dynamics. T(1)(-1) profiles obtained for 1-palmitoyl-2-(n-doxylstearoyl)phosphatidylcholine (n-PC) spin labels in dimyristoylphosphatidylcholine (DMPC) membranes with and without 50 mol% cholesterol are presented in parallel with profiles of the rotational diffusion coefficient, R(⊥), obtained from simulation of EPR spectra using Freed's model. These profiles are compared with profiles of the order parameter obtained directly from EPR spectra and with profiles of the order parameter obtained from simulation of EPR spectra. It is shown that T(1)(-1) and R(⊥) profiles reveal changes in membrane fluidity that depend on the motional properties of the lipid alkyl chain. We find that cholesterol has a rigidifying effect only to the depth occupied by the rigid steroid ring structure and a fluidizing effect at deeper locations. These effects cannot be differentiated by profiles of the order parameter. All profiles in this study were obtained at X-band (9.5 GHz).
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Marsh D. Electron spin resonance: spin labels. In: Grell E, editor. Membr. Spectrosc. Springer-Verlag; Berlin: 1981. pp. 51–142. - PubMed
-
- Devaux PF. ESR and NMR studies of lipid-protein interactions in membranes. In: Berliner LJ, Reuben J, editors. Biological Magnetic Resonance. Plenum Press; New York: 1983. pp. 183–299.
-
- Schreier S, Polnaszek CF, Smith IC. Spin labels in membranes: problems in practice. Biochim. Biophys. Acta. 1978;515:395–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

