Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin
- PMID: 21868357
- PMCID: PMC3415961
- DOI: 10.1126/scisignal.2001707
Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin
Abstract
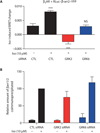
Phosphorylation of G protein-coupled receptors (GPCRs, which are also known as seven-transmembrane spanning receptors) by GPCR kinases (GRKs) plays essential roles in the regulation of receptor function by promoting interactions of the receptors with β-arrestins. These multifunctional adaptor proteins desensitize GPCRs, by reducing receptor coupling to G proteins and facilitating receptor internalization, and mediate GPCR signaling through β-arrestin-specific pathways. Detailed mapping of the phosphorylation sites on GPCRs targeted by individual GRKs and an understanding of how these sites regulate the specific functional consequences of β-arrestin engagement may aid in the discovery of therapeutic agents targeting individual β-arrestin functions. The β(2)-adrenergic receptor (β(2)AR) has many serine and threonine residues in the carboxyl-terminal tail and the intracellular loops, which are potential sites of phosphorylation. We monitored the phosphorylation of the β(2)AR at specific sites upon stimulation with an agonist that promotes signaling by both G protein-mediated and β-arrestin-mediated pathways or with a biased ligand that promotes signaling only through β-arrestin-mediated events in the presence of the full complement of GRKs or when either GRK2 or GRK6 was depleted. We correlated the specific and distinct patterns of receptor phosphorylation by individual GRKs with the functions of β-arrestins and propose that the distinct phosphorylation patterns established by different GRKs establish a "barcode" that imparts distinct conformations to the recruited β-arrestin, thus regulating its functional activities.
Figures



References
-
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. - PubMed
-
- Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through β-arrestin. Sci. STKE. 2005;2005:cm10. - PubMed
-
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. - PubMed
-
- Violin JD, Lefkowitz RJ. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007;28:416–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

