Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection
- PMID: 21868358
- PMCID: PMC3625704
- DOI: 10.1126/scisignal.2001748
Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection
Abstract
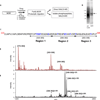
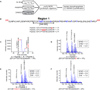
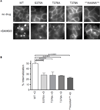
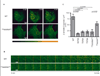
In comparison to endogenous ligands of seven-transmembrane receptors, which typically act as full agonists, many drugs act as partial agonists. Partial agonism is best described as a "macroscopic" property that is manifest at the level of physiological systems or cell populations; however, whether partial agonists also encode discrete regulatory information at the "microscopic" level of individual receptors is not known. Here, we addressed this question by focusing on morphine, a partial agonist drug for μ-type opioid peptide receptors (MORs), and by combining quantitative mass spectrometry with cell biological analysis to investigate the reduced efficacy of morphine, compared to that of a peptide full agonist, in promoting receptor endocytosis. We showed that these chemically distinct ligands produced a complex and qualitatively similar mixture of phosphorylated opioid receptor forms in intact cells. Quantitatively, however, the different agonists promoted disproportionate multisite phosphorylation of a specific serine and threonine motif, and we found that modification at more than one residue was essential for the efficient recruitment of the adaptor protein β-arrestin that mediated subsequent endocytosis of MORs. Thus, quantitative encoding of agonist-selective endocytosis at the level of individual opioid receptors was based on the conserved biochemical principles of multisite phosphorylation and threshold detection.
Figures



References
-
- Bradbury AF, Smyth DG, Snell CR. Biosynthetic origin and receptor conformation of methionine enkephalin. Nature. 1976 Mar 11;260:165. - PubMed
-
- Matthes HW, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene [see comments] Nature. 1996;383:819. - PubMed
-
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003 May 23;278:18776. - PubMed
-
- Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007 Oct;17:556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

