Dynamic remodeling of the actin cytoskeleton by FMNL1γ is required for structural maintenance of the Golgi complex
- PMID: 21868368
- PMCID: PMC3172187
- DOI: 10.1242/jcs.083725
Dynamic remodeling of the actin cytoskeleton by FMNL1γ is required for structural maintenance of the Golgi complex
Abstract
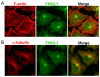
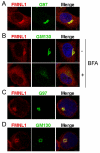
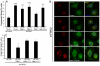
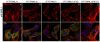
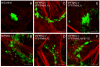
Formin-like 1 (FMNL1) is a member of the formin family of actin nucleators, and is one of the few formins for which in vitro activities have been well characterized. However, the functional roles of this mammalian formin remain ill-defined. In particular, it is unclear how the unique in vitro biochemical properties of FMNL1 relate to its regulation of cellular processes. Here, we demonstrate that FMNL1 depletion caused a dramatic increase in cellular F-actin content, which resulted in Golgi complex fragmentation. Moreover, increased F-actin and maintenance of Golgi structure were distinctly regulated by the gamma isoform of FMNL1, which required binding to actin. Importantly, in addition to Golgi fragmentation, increased F-actin content in the absence of FMNL1 also led to cation-independent mannose 6-phosphate receptor dispersal, lysosomal enlargement and missorting of cathepsin D. Taken together, our data support a model in which FMNL1 regulates cellular F-actin levels required to maintain structural integrity of the Golgi complex and lysosomes.
Figures


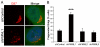
Similar articles
-
Bi-modal regulation of a formin by srGAP2.J Biol Chem. 2011 Feb 25;286(8):6577-86. doi: 10.1074/jbc.M110.190397. Epub 2010 Dec 9. J Biol Chem. 2011. PMID: 21148482 Free PMC article.
-
The carboxy-terminus of the formin FMNL1ɣ bundles actin to potentiate adenocarcinoma migration.J Cell Biochem. 2019 Sep;120(9):14383-14404. doi: 10.1002/jcb.28694. Epub 2019 Apr 11. J Cell Biochem. 2019. PMID: 30977161
-
RhoA-mediated FMNL1 regulates GM130 for actin assembly and phosphorylates MAPK for spindle formation in mouse oocyte meiosis.Cell Cycle. 2015;14(17):2835-43. doi: 10.1080/15384101.2015.1031438. Epub 2015 Jun 17. Cell Cycle. 2015. PMID: 26083584 Free PMC article.
-
Formins, Golgi, and the Centriole.Results Probl Cell Differ. 2019;67:27-48. doi: 10.1007/978-3-030-23173-6_3. Results Probl Cell Differ. 2019. PMID: 31435791 Review.
-
Mechanostress resistance involving formin homology proteins: G- and F-actin homeostasis-driven filament nucleation and helical polymerization-mediated actin polymer stabilization.Biochem Biophys Res Commun. 2018 Nov 25;506(2):323-329. doi: 10.1016/j.bbrc.2018.09.189. Epub 2018 Oct 9. Biochem Biophys Res Commun. 2018. PMID: 30309655 Review.
Cited by
-
Lysine acetylation of cytoskeletal proteins: Emergence of an actin code.J Cell Biol. 2020 Dec 7;219(12):e202006151. doi: 10.1083/jcb.202006151. J Cell Biol. 2020. PMID: 33044556 Free PMC article. Review.
-
Characterization of Leukocyte Formin FMNL1 Expression in Human Tissues.J Histochem Cytochem. 2014 Jun;62(6):460-470. doi: 10.1369/0022155414532293. Epub 2014 Apr 3. J Histochem Cytochem. 2014. PMID: 24700756 Free PMC article.
-
Human Macrophages Utilize the Podosome Formin FMNL1 for Adhesion and Migration.Cellbio (Irvine, Calif). 2015 Mar;4(1):1-11. doi: 10.4236/cellbio.2015.41001. Epub 2015 Mar 4. Cellbio (Irvine, Calif). 2015. PMID: 26942206 Free PMC article.
-
Small-molecule agonists of mammalian Diaphanous-related (mDia) formins reveal an effective glioblastoma anti-invasion strategy.Mol Biol Cell. 2015 Nov 1;26(21):3704-18. doi: 10.1091/mbc.E14-11-1502. Epub 2015 Sep 9. Mol Biol Cell. 2015. PMID: 26354425 Free PMC article.
-
The formin FMNL3 assembles plasma membrane protrusions that participate in cell-cell adhesion.Mol Biol Cell. 2015 Feb 1;26(3):467-77. doi: 10.1091/mbc.E14-07-1247. Epub 2014 Nov 26. Mol Biol Cell. 2015. PMID: 25428984 Free PMC article.
References
-
- Chhabra E. S., Higgs H. N. (2006). INF2 is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J. Biol. Chem. 281, 26754-26767 - PubMed
-
- Chhabra E. S., Higgs H. N. (2007). The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 9, 1110-1121 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

