Dexamethasone enhances 1alpha,25-dihydroxyvitamin D3 effects by increasing vitamin D receptor transcription
- PMID: 21868377
- PMCID: PMC3196110
- DOI: 10.1074/jbc.M111.244061
Dexamethasone enhances 1alpha,25-dihydroxyvitamin D3 effects by increasing vitamin D receptor transcription
Abstract
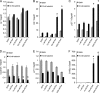
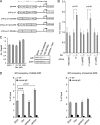
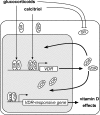
Calcitriol, the active form of vitamin D, in combination with the glucocorticoid dexamethasone (Dex) has been shown to increase the antitumor effects of calcitriol in squamous cell carcinoma. In this study we found that pretreatment with Dex potentiates calcitriol effects by inhibiting cell growth and increasing vitamin D receptor (VDR) and VDR-mediated transcription. Treatment with actinomycin D inhibits Vdr mRNA synthesis, indicating that Dex regulates VDR expression at transcriptional level. Real time PCR shows that treatment with Dex increases Vdr transcripts in a time- and a dose-dependent manner, indicating that Dex directly regulates expression of Vdr. RU486, an inhibitor of glucocorticoids, inhibits Dex-induced Vdr expression. In addition, the silencing of glucocorticoid receptor (GR) abolishes the induction of Vdr by Dex, indicating that Dex increases Vdr transcripts in a GR-dependent manner. A fragment located 5.2 kb upstream of Vdr transcription start site containing two putative glucocorticoid response elements (GREs) was evaluated using a luciferase-based reporter assay. Treatment with 100 nm Dex induces transcription of luciferase driven by the fragment. Deletion of the GRE distal to transcription start site was sufficient to abolish Dex induction of luciferase. Also, chromatin immunoprecipitation reveals recruitment of GR to distal GRE with Dex treatment. We conclude that Dex increases VDR and vitamin D effects by increasing Vdr de novo transcription in a GR-dependent manner.
Figures



References
-
- Dhawan P., Christakos S. (2010) J. Cell. Biochem. 110, 1314–1323 - PubMed
-
- Kallay E., Pietschmann P., Toyokuni S., Bajna E., Hahn P., Mazzucco K., Bieglmayer C., Kato S., Cross H. S. (2001) Carcinogenesis 22, 1429–1435 - PubMed
-
- Trump D. L., Potter D. M., Muindi J., Brufsky A., Johnson C. S. (2006) Cancer 106, 2136–2142 - PubMed
-
- Chung I., Karpf A. R., Muindi J. R., Conroy J. M., Nowak N. J., Johnson C. S., Trump D. L. (2007) J. Biol. Chem. 282, 8704–8714 - PubMed
-
- Chung I., Yu W. D., Karpf A. R., Flynn G., Bernardi R. J., Modzelewski R. A., Johnson C. S., Trump D. L. (2007) J. Steroid Biochem. Mol. Biol. 103, 768–770 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

