Reactions of beta-propiolactone with nucleobase analogues, nucleosides, and peptides: implications for the inactivation of viruses
- PMID: 21868382
- PMCID: PMC3196120
- DOI: 10.1074/jbc.M111.279232
Reactions of beta-propiolactone with nucleobase analogues, nucleosides, and peptides: implications for the inactivation of viruses
Abstract
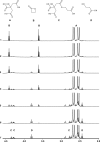
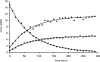
β-Propiolactone is often applied for inactivation of viruses and preparation of viral vaccines. However, the exact nature of the reactions of β-propiolactone with viral components is largely unknown. The purpose of the current study was to elucidate the chemical modifications occurring on nucleotides and amino acid residues caused by β-propiolactone. Therefore, a set of nucleobase analogues was treated with β-propiolactone, and reaction products were identified and quantified. NMR revealed at least one modification in either deoxyguanosine, deoxyadenosine, or cytidine after treatment with β-propiolactone. However, no reaction products were found from thymidine and uracil. The most reactive sides of the nucleobase analogues and nucleosides were identified by NMR. Furthermore, a series of synthetic peptides was used to determine the conversion of reactive amino acid residues by liquid chromatography-mass spectrometry. β-Propiolactone was shown to react with nine different amino acid residues. The most reactive residues are cysteine, methionine, and histidine and, to a lesser degree, aspartic acid, glutamic acid, tyrosine, lysine, serine, and threonine. Remarkably, cystine residues (disulfide groups) do not react with β-propiolactone. In addition, no reaction was observed for β-propiolactone with asparagine, glutamine, and tryptophan residues. β-Propiolactone modifies proteins to a larger extent than expected from current literature. In conclusion, the study determined the reactivity of β-propiolactone with nucleobase analogues, nucleosides, and amino acid residues and elucidated the chemical structures of the reaction products. The study provides detailed knowledge on the chemistry of β-propiolactone inactivation of viruses.
Figures
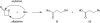
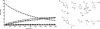





Similar articles
-
Surface modifications of influenza proteins upon virus inactivation by β-propiolactone.Proteomics. 2013 Dec;13(23-24):3537-47. doi: 10.1002/pmic.201300096. Proteomics. 2013. PMID: 24123778 Free PMC article.
-
Beta-Propiolactone Inactivation of Coxsackievirus A16 Induces Structural Alteration and Surface Modification of Viral Capsids.J Virol. 2017 Mar 29;91(8):e00038-17. doi: 10.1128/JVI.00038-17. Print 2017 Apr 15. J Virol. 2017. PMID: 28148783 Free PMC article.
-
Reaction of beta-propiolactone with amino acids and its specificity for methionine.Biochem J. 1968 Feb;106(4):829-34. doi: 10.1042/bj1060829. Biochem J. 1968. PMID: 5637366 Free PMC article.
-
Susceptibility of protein therapeutics to spontaneous chemical modifications by oxidation, cyclization, and elimination reactions.Amino Acids. 2019 Nov;51(10-12):1409-1431. doi: 10.1007/s00726-019-02787-2. Epub 2019 Oct 1. Amino Acids. 2019. PMID: 31576455 Review.
-
Inactivation of hepatitis viruses and HIV in plasma and plasma derivatives by treatment with beta-propiolactone/UV irradiation.Curr Stud Hematol Blood Transfus. 1989;(56):122-7. doi: 10.1159/000416562. Curr Stud Hematol Blood Transfus. 1989. PMID: 2642784 Review.
Cited by
-
Assessments of different inactivating reagents in formulating transmissible gastroenteritis virus vaccine.Virol J. 2020 Oct 23;17(1):163. doi: 10.1186/s12985-020-01433-8. Virol J. 2020. PMID: 33097081 Free PMC article.
-
Gamma-Irradiated Non-Capsule Group B Streptococcus Promotes T-Cell Dependent Immunity and Provides a Cross-Protective Reaction.Pharmaceuticals (Basel). 2023 Feb 20;16(2):321. doi: 10.3390/ph16020321. Pharmaceuticals (Basel). 2023. PMID: 37259463 Free PMC article.
-
Inactivated recombinant influenza vaccine: the promising direction for the next generation of influenza vaccine.Expert Rev Vaccines. 2024 Jan-Dec;23(1):409-418. doi: 10.1080/14760584.2024.2333338. Epub 2024 Mar 25. Expert Rev Vaccines. 2024. PMID: 38509022 Free PMC article. Review.
-
Structural and Immunoreactivity Properties of the SARS-CoV-2 Spike Protein upon the Development of an Inactivated Vaccine.Viruses. 2023 Feb 9;15(2):480. doi: 10.3390/v15020480. Viruses. 2023. PMID: 36851694 Free PMC article.
-
Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities.Vaccines (Basel). 2021 Dec 16;9(12):1490. doi: 10.3390/vaccines9121490. Vaccines (Basel). 2021. PMID: 34960236 Free PMC article. Review.
References
-
- Campbell T. M., Studdert M. J., Blackney M. H. (1982) Vet. Microbiol. 7, 535–544 - PubMed
-
- Frösner G. G., Stephan W., Dichtelmüller H. (1983) Eur. J. Clin. Microbiol. 2, 355–357 - PubMed
-
- Horowitz B. (1991) Biotechnology 19, 417–430 - PubMed
-
- Logrippo G. A. (1960) Ann. N.Y. Acad. Sci. 83, 578–594 - PubMed
-
- Race E., Stein C. A., Wigg M. D., Baksh A., Addawe M., Frezza P., Oxford J. S. (1995) Vaccine 13, 1567–1575 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

