Cathepsin L activity is essential to elastase perfusion-induced abdominal aortic aneurysms in mice
- PMID: 21868704
- PMCID: PMC3312037
- DOI: 10.1161/ATVBAHA.111.230201
Cathepsin L activity is essential to elastase perfusion-induced abdominal aortic aneurysms in mice
Abstract
Objective: The development of abdominal aortic aneurysms (AAA) requires extensive aortic wall matrix degradation. Human AAA lesions express high levels of cathepsin L (CatL), one of the most potent mammalian elastases. Whether this protease participates directly in AAA pathogenesis, however, is unknown.
Methods and results: We generated experimental AAA with aortic elastase perfusion in mice and established an essential role of CatL in AAA formation. After 14 days postperfusion, most wild-type (Ctsl(+/+)) mice developed AAA, but none of the CatL-deficient (Ctsl(-/-)) mice did. AAA lesion macrophage contents, CD4(+) T cell numbers, CD31(+) and laminin-5 angiogenic fragment γ2(+) microvessel numbers, and elastin fragmentation were all significantly lower in Ctsl(-/-) mice than in Ctsl(+/+) mice. While lesions from Ctsl(-/-) mice contained fewer Ki67(+) proliferating cells than did Ctsl(+/+) mice, the absence of CatL did not affect lesion apoptotic cell contents or medial smooth-muscle cell loss significantly. Mechanistic studies indicated that the absence of CatL reduced lesion chemokine monocyte chemotactic protein-1 content, macrophage and T-cell in vitro transmigration, and angiogenesis, and altered the expression and activities of matrix metalloproteinases and other cysteinyl cathepsins in inflammatory cells, vascular cells, and AAA lesions.
Conclusion: CatL contributes to AAA formation by promoting lesion inflammatory cell accumulation, angiogenesis, and protease expression.
Figures
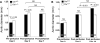





Similar articles
-
Cathepsin K deficiency reduces elastase perfusion-induced abdominal aortic aneurysms in mice.Arterioscler Thromb Vasc Biol. 2012 Jan;32(1):15-23. doi: 10.1161/ATVBAHA.111.235002. Epub 2011 Aug 4. Arterioscler Thromb Vasc Biol. 2012. PMID: 21817099 Free PMC article.
-
Deficiency of cathepsin S attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice.Cardiovasc Res. 2012 Dec 1;96(3):401-10. doi: 10.1093/cvr/cvs263. Epub 2012 Aug 7. Cardiovasc Res. 2012. PMID: 22871592 Free PMC article.
-
Tumor necrosis factor-like weak inducer of apoptosis or Fn14 deficiency reduce elastase perfusion-induced aortic abdominal aneurysm in mice.J Am Heart Assoc. 2014 Aug 4;3(4):e000723. doi: 10.1161/JAHA.113.000723. J Am Heart Assoc. 2014. PMID: 25092786 Free PMC article.
-
Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation.Circ Res. 2011 May 27;108(11):1316-27. doi: 10.1161/CIRCRESAHA.111.243758. Epub 2011 Apr 14. Circ Res. 2011. PMID: 21493897 Free PMC article.
-
Angiopoietins, abdominal aortic aneurysm and atherosclerosis.Atherosclerosis. 2011 Feb;214(2):237-43. doi: 10.1016/j.atherosclerosis.2010.08.051. Epub 2010 Aug 19. Atherosclerosis. 2011. PMID: 20832800 Free PMC article. Review.
Cited by
-
Role of cathepsin D activation in major adverse cardiovascular events and new-onset heart failure after STEMI.Herz. 2015 Sep;40(6):912-20. doi: 10.1007/s00059-015-4311-6. Epub 2015 Apr 25. Herz. 2015. PMID: 25911051 Clinical Trial.
-
Cathepsin L in tumor angiogenesis and its therapeutic intervention by the small molecule inhibitor KGP94.Clin Exp Metastasis. 2016 Jun;33(5):461-73. doi: 10.1007/s10585-016-9790-1. Epub 2016 Apr 7. Clin Exp Metastasis. 2016. PMID: 27055649 Free PMC article.
-
The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections.Eur J Clin Invest. 2022 Apr;52(4):e13697. doi: 10.1111/eci.13697. Epub 2021 Nov 21. Eur J Clin Invest. 2022. PMID: 34698377 Free PMC article. Review.
-
Cysteine protease cathepsins and matrix metalloproteinases in the development of abdominal aortic aneurysms.Future Cardiol. 2013 Jan;9(1):89-103. doi: 10.2217/fca.12.71. Future Cardiol. 2013. PMID: 23259477 Free PMC article. Review.
-
Plasma levels of cathepsins L, K, and V and risks of abdominal aortic aneurysms: a randomized population-based study.Atherosclerosis. 2013 Sep;230(1):100-105. doi: 10.1016/j.atherosclerosis.2013.05.018. Epub 2013 Jul 14. Atherosclerosis. 2013. PMID: 23958260 Free PMC article.
References
-
- Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of AAA progression. Part 1: extracellular matrix degeneration. Nat Rev Cardiol. 2009;6:464–474. - PubMed
-
- Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, Loskutoff DJ, Carmeliet P, Dole WP, Wang YX. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

