Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial
- PMID: 21868730
- PMCID: PMC3356690
- DOI: 10.1161/STROKEAHA.110.610949
Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial
Abstract
Background and purpose: Patients with intracerebral hemorrhage and intraventricular hemorrhage have a reported mortality of 50% to 80%. We evaluated a clot lytic treatment strategy for these patients in terms of mortality, ventricular infection, and bleeding safety events, and for its effect on the rate of intraventricular clot lysis.
Methods: Forty-eight patients were enrolled at 14 centers and randomized to treatment with 3 mg recombinant tissue-type plasminogen activator (rtPA) or placebo. Demographic characteristics, severity factors, safety outcomes (mortality, infection, bleeding), and clot resolution rates were compared in the 2 groups.
Results: Severity factors, including admission Glasgow Coma Scale, intracerebral hemorrhage volume, intraventricular hemorrhage volume, and blood pressure were evenly distributed, as were adverse events, except for an increased frequency of respiratory system events in the placebo-treated group. Neither intracranial pressure nor cerebral perfusion pressure differed substantially between treatment groups on presentation, with external ventricular device closure, or during the active treatment phase. Frequency of death and ventriculitis was substantially lower than expected and bleeding events remained below the prespecified threshold for mortality (18% rtPA; 23% placebo), ventriculitis (8% rtPA; 9% placebo), symptomatic bleeding (23% rtPA; 5% placebo, which approached statistical significance; P=0.1). The median duration of dosing was 7.5 days for rtPA and 12 days for placebo. There was a significant beneficial effect of rtPA on rate of clot resolution.
Conclusions: Low-dose rtPA for the treatment of intracerebral hemorrhage with intraventricular hemorrhage has an acceptable safety profile compared to placebo and historical controls. Data from a well-designed phase III clinical trial, such as CLEAR III, will be needed to fully evaluate this treatment.
Figures

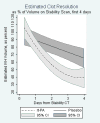
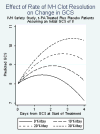
Comment in
-
CLEAR intraventricular hemorrhage: more than a glimmer of hope.Stroke. 2011 Nov;42(11):2999-3000. doi: 10.1161/STROKEAHA.111.628024. Epub 2011 Aug 25. Stroke. 2011. PMID: 21868731 No abstract available.
References
-
- Coplin WM, Vinas FC, Agris JM, Buciuc R, Michael DB, Diaz FG, et al. A cohort study of the safety and feasibility of intraventricular urokinase for nonaneurysmal spontaneous intraventricular hemorrhage. Stroke. 1998;29:1573–1579. - PubMed
-
- Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–621. - PubMed
-
- Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 2: In vivo safety study of intraventricular urokinase. Neurosurgery. 1986;19:547–552. - PubMed
-
- Wagner KR, Xi G, Hua Y, Zuccarello M, de Courten-Myers GM, Broderick JP, et al. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: Edema reduction and blood-brain barrier protection. J Neurosurg. 1999;90:491–498. - PubMed
-
- Mayfrank L, Kissler J, Raoofi R. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28:141–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

