Pharmacokinetics of modified slow-release oral testosterone over 9 days in normal men with experimental hypogonadism
- PMID: 21868746
- PMCID: PMC4034539
- DOI: 10.2164/jandrol.111.014514
Pharmacokinetics of modified slow-release oral testosterone over 9 days in normal men with experimental hypogonadism
Abstract
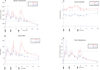
Oral administration of testosterone has potential use for the treatment of hypogonadism. We have recently demonstrated that a novel formulation of oral testosterone transiently normalized serum testosterone in a single-dose pharmacokinetic study. In this report, we present the steady-state pharmacokinetics of this formulation. Twelve healthy young men were rendered hypogonadal with the gonadotropin-releasing hormone antagonist acyline (300 μg/kg subcutaneously) and administered 300 mg of oral testosterone 3 times daily for 9 days. Serum testosterone, dihydrotestosterone (DHT), estradiol, and sex hormone-binding globulin (SHBG) were measured before and 1, 2, 4, 5, 6, 8, 10, 11, 12, 14, 16, and 24 hours on the first and ninth day of dosing. Before testosterone administration, all men had serum testosterone under 75 ng/dL. Over day 1, the 24-hour average (geometric mean [%CV]) serum total testosterone was 378 (45) ng/dL. This decreased to 315 (41) ng/dL after 9 days of continuous treatment (P = .1 compared with day 1). The 24-hour average serum SHBG was 27 (46) nmol/L on day 1 and was significantly reduced to 19 (47) nmol/L by day 9 (P < .01). As a result, the calculated free testosterone values were similar between day 1 and day 9: 8.7 (43) and 8.3 (37) ng/dL, respectively. DHT was in the reference range and estradiol was slightly below on day 9. Oral testosterone (300 mg) dosed 3 times daily normalized serum testosterone in men with experimentally induced hypogonadism after 9 days of dosing and significantly suppressed SHBG. This formulation of oral testosterone may have efficacy for the treatment of testosterone deficiency.
Figures
Similar articles
-
Pharmacokinetics of 2 novel formulations of modified-release oral testosterone alone and with finasteride in normal men with experimental hypogonadism.J Androl. 2010 Nov-Dec;31(6):527-35. doi: 10.2164/jandrol.109.009746. Epub 2010 Apr 8. J Androl. 2010. PMID: 20378927 Free PMC article. Clinical Trial.
-
Pharmacokinetics and pharmacodynamics of subcutaneous testosterone implants in hypogonadal men.Clin Endocrinol (Oxf). 1996 Jul;45(1):61-71. doi: 10.1111/j.1365-2265.1996.tb02061.x. Clin Endocrinol (Oxf). 1996. PMID: 8796140
-
Testosterone release from a subcutaneous, biodegradable microcapsule formulation (Viatrel) in hypogonadal men.J Androl. 2002 Jan-Feb;23(1):84-91. doi: 10.1002/jand.2002.23.1.84. J Androl. 2002. PMID: 11780927 Clinical Trial.
-
Diagnosis and Evaluation of Hypogonadism.Endocrinol Metab Clin North Am. 2022 Mar;51(1):47-62. doi: 10.1016/j.ecl.2021.11.001. Epub 2022 Feb 8. Endocrinol Metab Clin North Am. 2022. PMID: 35216720 Review.
-
JATENZO®: Challenges in the development of oral testosterone.Int J Impot Res. 2022 Nov;34(7):721-724. doi: 10.1038/s41443-021-00461-4. Epub 2021 Aug 5. Int J Impot Res. 2022. PMID: 34354245 Review.
Cited by
-
An oral lipidic native testosterone formulation that is absorbed independent of food.Eur J Endocrinol. 2021 Oct 5;185(5):607-615. doi: 10.1530/EJE-21-0606. Eur J Endocrinol. 2021. PMID: 34379604 Free PMC article.
-
Elevated Dihydrotestosterone is Associated with Testosterone Induced Erythrocytosis.J Urol. 2015 Jul;194(1):160-5. doi: 10.1016/j.juro.2015.01.038. Epub 2015 Jan 14. J Urol. 2015. PMID: 25596360 Free PMC article.
References
-
- Amory JK, Bremner WJ. Oral testosterone in oil plus dutasteride: a pharmacokinetic study in men. J Clin Endocrinol Metab. 2005;90:2610–2617. - PubMed
-
- Amory JK, Page ST, Bremner WJ. Oral testosterone in oil: pharmacokinetic effects of 5α-reduction with finasteride or dutasteride and food intake in men. J Androl. 2006;27:72–78. - PubMed
-
- Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS. REDUCE study group. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. - PubMed
-
- Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
