Loss of transcription factor KLF5 in the context of p53 ablation drives invasive progression of human squamous cell cancer
- PMID: 21868761
- PMCID: PMC3193554
- DOI: 10.1158/0008-5472.CAN-11-1702
Loss of transcription factor KLF5 in the context of p53 ablation drives invasive progression of human squamous cell cancer
Abstract
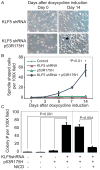
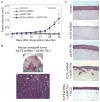
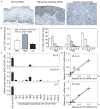
Squamous cell cancers account for more than half of all human cancers, and esophageal cancer is the sixth leading cause of cancer death worldwide. The majority of esophageal squamous cell carcinomas have identifiable p53 mutations, yet the same p53 mutations are found at comparable frequencies in precancerous dysplasia, indicating that transformation requires additional somatic changes yet to be defined. Here, we show that the zinc finger transcription factor Krüppel-like factor 5 (KLF5) transactivates NOTCH1 in the context of p53 mutation or loss. KLF5 loss limited NOTCH1 activity and was sufficient on its own to transform primary human keratinocytes harboring mutant p53, leading to the formation of invasive tumors. Restoration of NOTCH1 blocked transformation of KLF5-deficient and p53-mutant keratinocytes. Although human dysplastic epithelia accumulated KLF5, KLF5 expression was lost concurrently with NOTCH1 in squamous cell cancers. Taken together, these results define KLF5 loss as a critical event in squamous cell transformation and invasion. Our findings suggest that KLF5 may be a useful diagnostic and therapeutic target in esophageal squamous carcinomas and possibly more generally in other cancers associated with p53 loss of function.
Conflict of interest statement
Figures


Similar articles
-
p53 mutation alters the effect of the esophageal tumor suppressor KLF5 on keratinocyte proliferation.Cell Cycle. 2012 Nov 1;11(21):4033-9. doi: 10.4161/cc.22265. Epub 2012 Sep 18. Cell Cycle. 2012. PMID: 22990386 Free PMC article.
-
The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation.Genes Dev. 2007 Nov 1;21(21):2788-803. doi: 10.1101/gad.1544507. Genes Dev. 2007. PMID: 17974918 Free PMC article.
-
Overexpression of Kruppel-like factor 5 in esophageal epithelia in vivo leads to increased proliferation in basal but not suprabasal cells.Am J Physiol Gastrointest Liver Physiol. 2007 Jun;292(6):G1784-92. doi: 10.1152/ajpgi.00541.2006. Epub 2007 Mar 29. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17395897
-
KLF5, a Novel Therapeutic Target in Squamous Cell Carcinoma.DNA Cell Biol. 2021 Dec;40(12):1503-1512. doi: 10.1089/dna.2021.0674. DNA Cell Biol. 2021. PMID: 34931868 Review.
-
[The use of p53 as a tool for human cancer therapy].Mol Biol (Mosk). 2007 Nov-Dec;41(6):947-63. Mol Biol (Mosk). 2007. PMID: 18318112 Free PMC article. Review.
Cited by
-
miR-92a-3p promotes breast cancer proliferation by regulating the KLF2/BIRC5 axis.Thorac Cancer. 2022 Nov;13(21):2992-3000. doi: 10.1111/1759-7714.14648. Epub 2022 Sep 13. Thorac Cancer. 2022. PMID: 36100919 Free PMC article.
-
The roles and regulation of the KLF5 transcription factor in cancers.Cancer Sci. 2021 Jun;112(6):2097-2117. doi: 10.1111/cas.14910. Epub 2021 May 3. Cancer Sci. 2021. PMID: 33811715 Free PMC article. Review.
-
KLF4 activates NFκB signaling and esophageal epithelial inflammation via the Rho-related GTP-binding protein RHOF.PLoS One. 2019 Apr 18;14(4):e0215746. doi: 10.1371/journal.pone.0215746. eCollection 2019. PLoS One. 2019. PMID: 30998758 Free PMC article.
-
miR-320-3p regulates the proliferation, migration and apoptosis of hypoxia-induced pulmonary arterial smooth muscle cells via KLF5 and HIF1α.Am J Transl Res. 2021 Apr 15;13(4):2283-2295. eCollection 2021. Am J Transl Res. 2021. PMID: 34017389 Free PMC article.
-
KLF5 regulates epithelial-mesenchymal transition of liver cancer cells in the context of p53 loss through miR-192 targeting of ZEB2.Cell Adh Migr. 2020 Dec;14(1):182-194. doi: 10.1080/19336918.2020.1826216. Cell Adh Migr. 2020. PMID: 32965165 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010 - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–46. - PubMed
-
- Mandard AM, Hainaut P, Hollstein M. Genetic steps in the development of squamous cell carcinoma of the esophagus. Mutat Res. 2000;462:335–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

