Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk
- PMID: 21868778
- PMCID: PMC3161284
- DOI: 10.2337/dc10-2411
Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk
Abstract
Objective: To evaluate a sensor-augmented insulin pump with a low glucose suspend (LGS) feature that automatically suspends basal insulin delivery for up to 2 h in response to sensor-detected hypoglycemia.
Research design and methods: The LGS feature of the Paradigm Veo insulin pump (Medtronic, Inc., Northridge, CA) was tested for 3 weeks in 31 adults with type 1 diabetes.
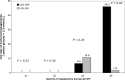
Results: There were 166 episodes of LGS: 66% of daytime LGS episodes were terminated within 10 min, and 20 episodes lasted the maximum 2 h. LGS use was associated with reduced nocturnal duration ≤2.2 mmol/L in those in the highest quartile of nocturnal hypoglycemia at baseline (median 46.2 vs. 1.8 min/day, P = 0.02 [LGS-OFF vs. LGS-ON]). Median sensor glucose was 3.9 mmol/L after 2-h LGS and 8.2 mmol/L at 2 h after basal restart.
Conclusions: Use of an insulin pump with LGS was associated with reduced nocturnal hypoglycemia in those at greatest risk and was well accepted by patients.
Trial registration: ClinicalTrials.gov NCT01267175.
Figures
Comment in
-
Closing the loop: another step forward.Diabetes Care. 2011 Sep;34(9):2136-7. doi: 10.2337/dc11-1217. Diabetes Care. 2011. PMID: 21868784 Free PMC article. No abstract available.
References
-
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 - PubMed
-
- O’Connell MA, Donath S, O’Neal DN, et al. . Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 2009;52:1250–1257 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous