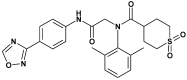
Effect of ASP2151, a herpesvirus helicase-primase inhibitor, in a guinea pig model of genital herpes
- PMID: 21869749
- PMCID: PMC6264763
- DOI: 10.3390/molecules16097210
Effect of ASP2151, a herpesvirus helicase-primase inhibitor, in a guinea pig model of genital herpes
Abstract
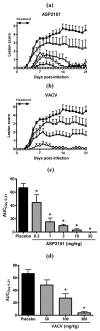
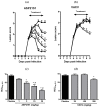
ASP2151 is a herpesvirus helicase-primase inhibitor with antiviral activity against varicella zoster virus and herpes simplex virus types 1 (HSV-1) and 2 (HSV-2). Here, we examined the potency and efficacy of ASP2151 against HSV in vitro and in vivo. We found that ASP2151 was more potent in inhibiting the replication of HSV-1 and HSV-2 in Vero cells in the plaque reduction assay and had greater anti-HSV activity in a guinea pig model of genital herpes than did acyclovir and valacyclovir (VACV), respectively. Oral ASP2151 given from the day of infection reduced peak and overall disease scores in a dose-dependent manner, resulting in complete prevention of symptoms at the dose of 30 mg/kg. The 50% effective dose (ED(50)) values for ASP2151 and VACV were 0.37 and 68 mg/kg, respectively, indicating that ASP2151 was 184-fold more potent than VACV. When ASP2151 was administered after the onset of symptoms, the disease course of genital herpes was suppressed more effectively than by VACV, with a significant reduction in disease score observed one day after starting ASP2151 at 30 mg/kg, whereas the therapeutic effect of VACV was only evident three days after treatment at the highest dose tested (300 mg/kg). This indicated that ASP2151 possesses a faster onset of action and wider therapeutic time window than VACV. Further, virus shedding from the genital mucosa was significantly reduced with ASP2151 at 10 and 30 mg/kg but not with VACV, even at 300 mg/kg. Taken together, our present findings demonstrated the superior potency and efficacy of ASP2151 against HSV.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pellet P.E., Roizman B. The family Herpesviridae: A brief introduction. In: Knipe D., Howley P.M., editors. Fields Virology. 5th. Vol. 2. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 2479–2499.
-
- Benedetti J., Corey L., Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann. Intern. Med. 1994;121:847–854. - PubMed
-
- Kinghorn G.R. Epidemiology of genital herpes. J. Int. Med. Res. 1994;22(Suppl. 1):14–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

