Neoadjuvant in situ gene-mediated cytotoxic immunotherapy improves postoperative outcomes in novel syngeneic esophageal carcinoma models
- PMID: 21869822
- PMCID: PMC3215998
- DOI: 10.1038/cgt.2011.56
Neoadjuvant in situ gene-mediated cytotoxic immunotherapy improves postoperative outcomes in novel syngeneic esophageal carcinoma models
Abstract
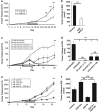
Esophageal carcinoma is the most rapidly increasing tumor in the United States and has a dismal 15% 5-year survival. Immunotherapy has been proposed to improve patient outcomes; however, no immunocompetent esophageal carcinoma model exists to date to test this approach. We developed two mouse models of esophageal cancer by inoculating immunocompetent mice with syngeneic esophageal cell lines transformed by cyclin-D1 or mutant HRAS(G12V) and loss of p53. Similar to humans, surgery and adjuvant chemotherapy (cisplatin and 5-fluorouracil) demonstrated limited efficacy. Gene-mediated cyototoxic immunotherapy (adenoviral vector carrying the herpes simplex virus thymidine kinase gene in combination with the prodrug ganciclovir; AdV-tk/GCV) demonstrated high levels of in vitro transduction and efficacy. Using in vivo syngeneic esophageal carcinoma models, combining surgery, chemotherapy and AdV-tk/GCV improved survival (P=0.007) and decreased disease recurrence (P<0.001). Mechanistic studies suggested that AdV-tk/GCV mediated a direct cytotoxic effect and an increased intra-tumoral trafficking of CD8 T cells (8.15% vs 14.89%, P=0.02). These data provide the first preclinical evidence that augmenting standard of care with immunotherapy may improve outcomes in the management of esophageal carcinoma.
Figures


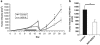


Similar articles
-
Adenovirus vector-mediated herpes simplex virus-thymidine kinase gene/ganciclovir system exhibits anti-tumor effects in an orthotopic hepatocellular carcinoma model.Pharmazie. 2014 Jul;69(7):547-52. Pharmazie. 2014. PMID: 25073402
-
Adenovirus-mediated suicide gene therapy for experimental bladder cancer.Urology. 1997 Feb;49(2):173-80. doi: 10.1016/S0090-4295(96)00560-2. Urology. 1997. PMID: 9037277
-
Neoadjuvant Gene-Mediated Cytotoxic Immunotherapy for Non-Small-Cell Lung Cancer: Safety and Immunologic Activity.Mol Ther. 2021 Feb 3;29(2):658-670. doi: 10.1016/j.ymthe.2020.11.001. Epub 2020 Nov 5. Mol Ther. 2021. PMID: 33160076 Free PMC article.
-
Prospects for herpes-simplex-virus thymidine-kinase and cytokine gene transduction as immunomodulatory gene therapy for prostate cancer.World J Urol. 2000 Apr;18(2):130-5. doi: 10.1007/s003450050185. World J Urol. 2000. PMID: 10854148 Review.
-
A narrative review on advances in neoadjuvant immunotherapy for esophageal cancer: Molecular biomarkers and future directions.Int J Cancer. 2025 Jan 1;156(1):20-33. doi: 10.1002/ijc.35153. Epub 2024 Sep 14. Int J Cancer. 2025. PMID: 39276114 Review.
Cited by
-
Intraoperative near-infrared imaging of surgical wounds after tumor resections can detect residual disease.Clin Cancer Res. 2012 Oct 15;18(20):5741-51. doi: 10.1158/1078-0432.CCR-12-1188. Epub 2012 Aug 29. Clin Cancer Res. 2012. PMID: 22932668 Free PMC article.
-
Andrographis paniculata elicits anti-invasion activities by suppressing TM4SF3 gene expression and by anoikis-sensitization in esophageal cancer cells.Am J Cancer Res. 2015 Nov 15;5(12):3570-87. eCollection 2015. Am J Cancer Res. 2015. PMID: 26885447 Free PMC article.
-
The adjuvant value of Andrographis paniculata in metastatic esophageal cancer treatment - from preclinical perspectives.Sci Rep. 2017 Apr 12;7(1):854. doi: 10.1038/s41598-017-00934-x. Sci Rep. 2017. PMID: 28405006 Free PMC article.
-
Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer.Cell Death Differ. 2014 Jan;21(1):15-25. doi: 10.1038/cdd.2013.67. Epub 2013 Jun 21. Cell Death Differ. 2014. PMID: 23787994 Free PMC article. Review.
-
Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma.Cancer Immunol Immunother. 2015 Jun;64(6):727-36. doi: 10.1007/s00262-015-1679-3. Epub 2015 Mar 21. Cancer Immunol Immunother. 2015. PMID: 25795132 Free PMC article. Clinical Trial.
References
-
- NCI Surveillance, Epidemiology, and End Results (SEER) ProgramNov 2009 Sub (1973–2007) <Katrian/Rita Population Adjustment> edn.SEER Stat Database: Bethesda, MD; 2009
-
- Berger AC, Farma J, Scott WJ, Freedman G, Weiner L, Cheng JD, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–4337. - PubMed
-
- Juergens RA, Forastiere A.Combined modality therapy of esophageal cancer J Natl Compr Canc Netw 20086851–860.quiz 861. - PubMed
-
- Zhang X, Watson DI, Jamieson GG, Bessell JR, Devitt PG. Neoadjuvant chemoradiotherapy for esophageal carcinoma. Dis Esophagus. 2005;18:104–108. - PubMed
-
- Neves S, Faneca H, Bertin S, Konopka K, Duzgunes N, Pierrefite-Carle V, et al. Transferrin lipoplex-mediated suicide gene therapy of oral squamous cell carcinoma in an immunocompetent murine model and mechanisms involved in the antitumoral response. Cancer Gene Ther. 2009;16:91–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

