L-selectin and SDF-1 enhance the migration of mouse and human cardiac mesoangioblasts
- PMID: 21869829
- PMCID: PMC3263491
- DOI: 10.1038/cdd.2011.110
L-selectin and SDF-1 enhance the migration of mouse and human cardiac mesoangioblasts
Abstract
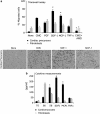
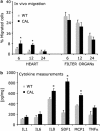
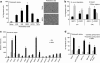
Efficient delivery of stem cells to heart regions is still a major problem for cell therapy. Here, we report experiments aimed to improve migration of mouse and human cardiac mesoangioblasts to the damaged heart. Cardiac mesoangioblasts were induced to transmigrate through the endothelium by factors released by cardiomyocytes or cytokines, among which stromal-derived factor 1 (SDF-1) was the most potent. Cardiac mesoangioblasts were also delivered into the left ventricular (LV) chamber of mice after coronary artery ligation (CAL), and their in vivo homing to the damaged heart was found to be quite modest. Pretreatment of cardiac mesoangioblasts with SDF-1 or transient expression of L-selectin induced a two- to three-fold increase in their transmigration and homing to the damaged heart. Therefore, combined pretreatment with SDF-1 and L-selectin generated modified cardiac mesoangioblasts, 50% of which, after injection into the LV chamber of mice early after CAL, home directly to the damaged free wall of the heart. Finally, modified mouse cardiac mesoangioblasts, injected into the LV chamber regenerate a larger surface of the ventricle in long-term experiments in comparison with their control counterparts. This study defines the requirements for efficient homing of cardiac mesoangioblasts to the damaged heart and offers a new potent tool to optimize efficiency of future cell therapy protocols for cardiovascular diseases.
Figures



Similar articles
-
Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability.J Cell Biol. 2006 Jul 17;174(2):231-43. doi: 10.1083/jcb.200512085. Epub 2006 Jul 10. J Cell Biol. 2006. PMID: 16831885 Free PMC article.
-
Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction.Am J Physiol Heart Circ Physiol. 2012 Sep 1;303(5):H605-18. doi: 10.1152/ajpheart.00366.2012. Epub 2012 Jun 29. Am J Physiol Heart Circ Physiol. 2012. PMID: 22752632 Free PMC article.
-
Cardiac mesoangioblasts are committed, self-renewable progenitors, associated with small vessels of juvenile mouse ventricle.Cell Death Differ. 2008 Sep;15(9):1417-28. doi: 10.1038/cdd.2008.75. Epub 2008 May 23. Cell Death Differ. 2008. PMID: 18497758
-
Overexpression of SDF-1α enhanced migration and engraftment of cardiac stem cells and reduced infarcted size via CXCR4/PI3K pathway.PLoS One. 2012;7(9):e43922. doi: 10.1371/journal.pone.0043922. Epub 2012 Sep 11. PLoS One. 2012. PMID: 22984452 Free PMC article.
-
Chemokine stromal cell-derived factor 1/CXCL12 increases homing of mesenchymal stem cells to injured myocardium and neovascularization following myocardial infarction.Chin Med J (Engl). 2009 Jan 20;122(2):183-7. doi: 10.3901/jme.2009.07.183. Chin Med J (Engl). 2009. PMID: 19187644
Cited by
-
Stem Cell Homing in Intrathecal Applications and Inspirations for Improvement Paths.Int J Mol Sci. 2022 Apr 13;23(8):4290. doi: 10.3390/ijms23084290. Int J Mol Sci. 2022. PMID: 35457107 Free PMC article. Review.
-
The potential of stem cells in the treatment of cardiovascular diseases.Stem Cell Rev Rep. 2013 Dec;9(6):814-32. doi: 10.1007/s12015-013-9461-4. Stem Cell Rev Rep. 2013. PMID: 23949886 Free PMC article. Review.
-
Method for obtaining committed adult mesenchymal precursors from skin and lung tissue.PLoS One. 2012;7(12):e53215. doi: 10.1371/journal.pone.0053215. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300894 Free PMC article.
-
Gradient biomaterials and their influences on cell migration.Interface Focus. 2012 Jun 6;2(3):337-55. doi: 10.1098/rsfs.2011.0124. Epub 2012 Mar 21. Interface Focus. 2012. PMID: 23741610 Free PMC article.
-
Stem cells in thoracic aortic aneurysms and dissections: potential contributors to aortic repair.Ann Thorac Surg. 2012 May;93(5):1524-33. doi: 10.1016/j.athoracsur.2012.01.063. Epub 2012 Mar 20. Ann Thorac Surg. 2012. PMID: 22440369 Free PMC article.
References
-
- Butcher E. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed
-
- Fu S, Liesveld J. Mobilization of hematopoietic stem cells. Blood Rev. 2000;14:205–218. - PubMed
-
- Grabovsky V, Feigelson S, Chen C, Bleijs D, Peled A, Cinamon G, et al. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med. 2000;192:495–506. - PMC - PubMed
-
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. - PubMed
-
- Springer T. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

