Molecular Imaging of Healing After Myocardial Infarction
- PMID: 21869911
- PMCID: PMC3159171
- DOI: 10.1007/s12410-010-9058-0
Molecular Imaging of Healing After Myocardial Infarction
Abstract
The progression from acute myocardial infarction (MI) to heart failure continues to be a major cause of morbidity and mortality. Potential new therapies for improved infarct healing such as stem cells, gene therapy, and tissue engineering are being investigated. Noninvasive imaging plays a central role in the evaluation of MI and infarct healing, both clinically and in preclinical research. Traditionally, imaging has been used to assess cardiac structure, function, perfusion, and viability. However, new imaging methods can be used to assess biological processes at the cellular and molecular level. We review molecular imaging techniques for evaluating the biology of infarct healing and repair. Specifically, we cover recent advances in imaging the various phases of MI and infarct healing such as apoptosis, inflammation, angiogenesis, extracellular matrix deposition, and scar formation. Significant progress has been made in preclinical molecular imaging, and future challenges include translation of these methods to clinical practice.
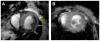
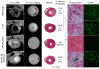
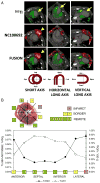
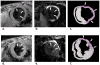
Figures



Similar articles
-
Role of osteopontin in heart failure associated with aging.Heart Fail Rev. 2010 Sep;15(5):487-94. doi: 10.1007/s10741-010-9158-6. Heart Fail Rev. 2010. PMID: 20127409 Review.
-
Modifying the mechanics of healing infarcts: Is better the enemy of good?J Mol Cell Cardiol. 2016 Apr;93:115-24. doi: 10.1016/j.yjmcc.2015.11.028. Epub 2015 Nov 26. J Mol Cell Cardiol. 2016. PMID: 26631496 Free PMC article. Review.
-
Lgr4 Governs a Pro-Inflammatory Program in Macrophages to Antagonize Post-Infarction Cardiac Repair.Circ Res. 2020 Sep 25;127(8):953-973. doi: 10.1161/CIRCRESAHA.119.315807. Epub 2020 Jun 30. Circ Res. 2020. PMID: 32600176
-
Noninvasive Assessment of an Engineered Bioactive Graft in Myocardial Infarction: Impact on Cardiac Function and Scar Healing.Stem Cells Transl Med. 2017 Feb;6(2):647-655. doi: 10.5966/sctm.2016-0063. Epub 2016 Sep 2. Stem Cells Transl Med. 2017. PMID: 28191775 Free PMC article.
-
Nuclear Imaging of Post-infarction Inflammation in Ischemic Cardiac Diseases - New Radiotracers for Potential Clinical Applications.Curr Radiopharm. 2021;14(3):184-208. doi: 10.2174/1874471013666201012165305. Curr Radiopharm. 2021. PMID: 33045975 Review.
Cited by
-
Cardiac PET, CT, and MR: what are the advantages of hybrid imaging?Curr Cardiol Rep. 2012 Feb;14(1):24-31. doi: 10.1007/s11886-011-0231-0. Curr Cardiol Rep. 2012. PMID: 22090194
-
Spectral CT imaging of myocardial infarction: preliminary animal experience.Eur Radiol. 2013 Jan;23(1):133-8. doi: 10.1007/s00330-012-2560-9. Epub 2012 Jul 20. Eur Radiol. 2013. PMID: 22814826
-
MR Assessment of Acute Pathologic Process after Myocardial Infarction in a Permanent Ligation Mouse Model: Role of Magnetic Nanoparticle-Contrasted MRI.Contrast Media Mol Imaging. 2017 Oct 18;2017:2870802. doi: 10.1155/2017/2870802. eCollection 2017. Contrast Media Mol Imaging. 2017. PMID: 29114174 Free PMC article.
References
-
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. - PubMed
-
- Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105(2):151–63. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
