Nonimmune Cells Contribute to Crosstalk between Immune Cells and Inflammatory Mediators in the Innate Response to Trypanosoma cruzi Infection
- PMID: 21869919
- PMCID: PMC3159004
- DOI: 10.1155/2012/737324
Nonimmune Cells Contribute to Crosstalk between Immune Cells and Inflammatory Mediators in the Innate Response to Trypanosoma cruzi Infection
Abstract
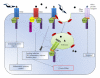
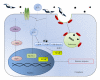
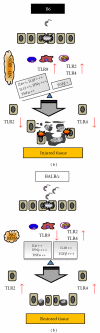
Chagas myocarditis, which is caused by infection with the intracellular parasite Trypanosoma cruzi, remains the major infectious heart disease worldwide. Innate recognition through toll-like receptors (TLRs) on immune cells has not only been revealed to be critical for defense against T. cruzi but has also been involved in triggering the pathology. Subsequent studies revealed that this parasite activates nucleotide-binding oligomerization domain- (NOD-)like receptors and several particular transcription factors in TLR-independent manner. In addition to professional immune cells, T. cruzi infects and resides in different parenchyma cells. The innate receptors in nonimmune target tissues could also have an impact on host response. Thus, the outcome of the myocarditis or the inflamed liver relies on an intricate network of inflammatory mediators and signals given by immune and nonimmune cells. In this paper, we discuss the evidence of innate immunity to the parasite developed by the host, with emphasis on the crosstalk between immune and nonimmune cell responses.
Figures
Similar articles
-
Virulence of Trypanosoma cruzi Strains Is Related to the Differential Expression of Innate Immune Receptors in the Heart.Front Cell Infect Microbiol. 2021 Jul 15;11:696719. doi: 10.3389/fcimb.2021.696719. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34336720 Free PMC article.
-
NFATc1 mediates Toll-like receptor-independent innate immune responses during Trypanosoma cruzi infection.PLoS Pathog. 2009 Jul;5(7):e1000514. doi: 10.1371/journal.ppat.1000514. Epub 2009 Jul 17. PLoS Pathog. 2009. PMID: 19609356 Free PMC article.
-
The Blockade of Interleukin-2 During the Acute Phase of Trypanosoma cruzi Infection Reveals Its Dominant Regulatory Role.Front Cell Infect Microbiol. 2021 Nov 17;11:758273. doi: 10.3389/fcimb.2021.758273. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34869064 Free PMC article.
-
Role of the Complement System in the Modulation of T-Cell Responses in Chronic Chagas Disease.Front Cell Infect Microbiol. 2022 Jun 30;12:910854. doi: 10.3389/fcimb.2022.910854. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35846776 Free PMC article. Review.
-
Trypanosoma cruzi and its components as exogenous mediators of inflammation recognized through Toll-like receptors.Mediators Inflamm. 2004 Jun;13(3):139-43. doi: 10.1080/09511920410001713565. Mediators Inflamm. 2004. PMID: 15223603 Free PMC article. Review.
Cited by
-
TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review.Braz J Infect Dis. 2016 Mar-Apr;20(2):193-204. doi: 10.1016/j.bjid.2015.10.011. Epub 2016 Jan 14. Braz J Infect Dis. 2016. PMID: 26775799 Free PMC article. Review.
-
Differential Activation of Human Monocytes and Lymphocytes by Distinct Strains of Trypanosoma cruzi.PLoS Negl Trop Dis. 2015 Jul 6;9(7):e0003816. doi: 10.1371/journal.pntd.0003816. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26147698 Free PMC article.
-
Zileuton, a 5-Lypoxigenase Inhibitor, is Antiparasitic and Prevents Inflammation in the Chronic Stage of Heart Chagas Disease.ACS Infect Dis. 2024 Dec 13;10(12):4258-4270. doi: 10.1021/acsinfecdis.4c00623. Epub 2024 Nov 28. ACS Infect Dis. 2024. PMID: 39609255 Free PMC article.
-
Co-infection with distinct Trypanosoma cruzi strains induces an activated immune response in human monocytes.Parasite Immunol. 2019 Nov;41(11):e12668. doi: 10.1111/pim.12668. Epub 2019 Sep 30. Parasite Immunol. 2019. PMID: 31494949 Free PMC article.
-
TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration.Am J Physiol Gastrointest Liver Physiol. 2015 Jul 1;309(1):G30-41. doi: 10.1152/ajpgi.00031.2015. Epub 2015 Apr 30. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25930080 Free PMC article.
References
-
- WHO. Chagas disease (American trypanosomiasis) Fact sheet, no 340, 2010. - PubMed
-
- Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Tropica. 2010;115(1-2):14–21. - PubMed
-
- Gironès NU, Fresno M. Etiology of Chagas disease myocarditis: autoimmunity, parasite persistence, or both? Trends in Parasitology. 2003;19(1):19–22. - PubMed
-
- Leon JS, Engman DM. The significance of autoimmunity in the pathogenesis of chagas heart disease. Frontiers in Bioscience. 2003;8:e316–e323. - PubMed
-
- Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115(9):1109–1123. - PubMed

