Clozapine protects dopaminergic neurons from inflammation-induced damage by inhibiting microglial overactivation
- PMID: 21870076
- PMCID: PMC3633602
- DOI: 10.1007/s11481-011-9309-0
Clozapine protects dopaminergic neurons from inflammation-induced damage by inhibiting microglial overactivation
Abstract
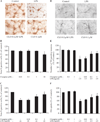
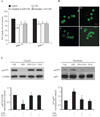
Increasing evidence suggests a possible involvement of neuroinflammation in some psychiatric disorders, and also pharmacological reports indicate that anti-inflammatory effects are associated with therapeutic actions of psychoactive drugs, such as anti-depressants and antipsychotics. The purpose of this study was to explore whether clozapine, a widely used antipsychotic drugs, displays anti-inflammatory and neuroprotective effects. Using primary cortical and mesencephalic neuron-glia cultures, we found that clozapine was protective against inflammation-related neurodegeneration induced by lipopolysaccharide (LPS). Pretreatment of cortical or mesencephalic neuron-glia cultures with clozapine (0.1 or 1 μM) for 24 h attenuated LPS-induced neurotoxicity. Clozapine also protected neurons against 1-methyl-4-phenylpyridinium(+) (MPP(+))-induced neurotoxicity, but only in cultures containing microglia, indicating an indispensable role of microglia in clozapine-afforded neuroprotection. Further observation revealed attenuated LPS-induced microglial activation in primary neuron-glia cultures and in HAPI microglial cell line with clozapine pretreatment. Clozapine ameliorated the production of microglia-derived superoxide and intracellular reactive oxygen species (ROS), as well as the production of nitric oxide and TNF-α following LPS. In addition, the protective effect of clozapine was not observed in neuron-glia cultures from mice lacking functional NADPH oxidase (PHOX), a key enzyme for superoxide production in immune cells. Further mechanistic studies demonstrated that clozapine pretreatment inhibited LPS-induced translocation of cytosolic subunit p47(phox) to the membrane in microglia, which was most likely through inhibiting the phosphoinositide 3-kinase (PI3K) pathway. Taken together, this study demonstrates that clozapine exerts neuroprotective effect via the attenuation of microglia activation through inhibition of PHOX-generated ROS production and suggests potential use of antipsychotic drugs for neuroprotection.
Conflict of interest statement
The authors declare that they have nocompeting interests or conflicts of interest.
Figures




References
-
- Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE. The hippocampus and parahippocampus in schizophrenia, suicide, and control brains. Arch Gen Psychiatry. 1990;47:1029–1034. - PubMed
-
- Anderson KE, Boyle KB, Davidson K, Chessa TA, Kulkarni S, Jarvis GE, Sindrilaru A, Scharffetter-Kochanek K, Rausch O, Stephens LR, Hawkins PT. CD18-dependent activation of the neutrophil NADPH oxidase during phagocytosis of Escherichia coli or Staphylococcus aureus is regulated by class III but not class I or II PI3Ks. Blood. 2008;112:5202–5211. - PubMed
-
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

