Fucose content of monoclonal antibodies can be controlled by culture medium osmolality for high antibody-dependent cellular cytotoxicity
- PMID: 21870215
- PMCID: PMC3386387
- DOI: 10.1007/s10616-011-9377-2
Fucose content of monoclonal antibodies can be controlled by culture medium osmolality for high antibody-dependent cellular cytotoxicity
Abstract
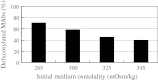
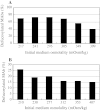
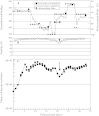
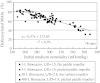
Antibody-dependent cellular cytotoxicity (ADCC) is dependent on the fucose content of oligosaccharides bound to monoclonal antibodies (MAbs). As MAbs with a low fucose content exhibit high ADCC activity, it is important to control the defucosylation levels (deFuc%) of MAbs and to analyze the factors that affect deFuc%. In this study, we observed that the deFuc% was inversely related to culture medium osmolality for MAbs produced in the rat hybridoma cell line YB2/0, with r (2) values as high as 0.92. Moreover, deFuc% exhibited the same correlation irrespective of the type of compound used for regulating osmolality (NaCl, KCl, fucose, fructose, creatine, or mannitol) at a culture scale ranging from 1 to 400 L. We succeeded in controlling MAb deFuc% by maintaining a constant medium osmolality in both perfusion and fed-batch cultures. In agreement with these observations, reverse transcription PCR analyses revealed decreased transcription of genes involved in glycolysis, GDP-fucose supply, and fucose transfer under hypoosmotic conditions.
Figures


Similar articles
-
Comparison of cell lines for stable production of fucose-negative antibodies with enhanced ADCC.Biotechnol Bioeng. 2006 Jul 5;94(4):680-8. doi: 10.1002/bit.20880. Biotechnol Bioeng. 2006. PMID: 16609957
-
The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity.J Biol Chem. 2003 Jan 31;278(5):3466-73. doi: 10.1074/jbc.M210665200. Epub 2002 Nov 8. J Biol Chem. 2003. PMID: 12427744
-
A novel bicistronic gene design couples stable cell line selection with a fucose switch in a designer CHO host to produce native and afucosylated glycoform antibodies.MAbs. 2018 Apr;10(3):416-430. doi: 10.1080/19420862.2018.1433975. Epub 2018 Feb 22. MAbs. 2018. PMID: 29400603 Free PMC article.
-
[Potelligent antibodies as next generation therapeutic antibodies].Yakugaku Zasshi. 2009 Jan;129(1):3-9. doi: 10.1248/yakushi.129.3. Yakugaku Zasshi. 2009. PMID: 19122430 Review. Japanese.
-
Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins.J Pharm Sci. 2015 Jun;104(6):1866-1884. doi: 10.1002/jps.24444. Epub 2015 Apr 14. J Pharm Sci. 2015. PMID: 25872915 Review.
Cited by
-
Osmolality Effects on CHO Cell Growth, Cell Volume, Antibody Productivity and Glycosylation.Int J Mol Sci. 2021 Mar 24;22(7):3290. doi: 10.3390/ijms22073290. Int J Mol Sci. 2021. PMID: 33804825 Free PMC article.
-
Antibody Structure and Function: The Basis for Engineering Therapeutics.Antibodies (Basel). 2019 Dec 3;8(4):55. doi: 10.3390/antib8040055. Antibodies (Basel). 2019. PMID: 31816964 Free PMC article. Review.
-
Monoclonal Antibody-Based Treatments for Neuromyelitis Optica Spectrum Disorders: From Bench to Bedside.Neurosci Bull. 2020 Oct;36(10):1213-1224. doi: 10.1007/s12264-020-00525-3. Epub 2020 Jun 12. Neurosci Bull. 2020. PMID: 32533450 Free PMC article. Review.
-
The Evolution of Anti-CD20 Treatment for Multiple Sclerosis: Optimization of Antibody Characteristics and Function.CNS Drugs. 2025 Jun;39(6):545-564. doi: 10.1007/s40263-025-01182-8. Epub 2025 Apr 3. CNS Drugs. 2025. PMID: 40180777 Free PMC article. Review.
-
A Milestone in Multiple Sclerosis Therapy: Monoclonal Antibodies Against CD20-Yet Progress Continues.Neurotherapeutics. 2021 Jul;18(3):1602-1622. doi: 10.1007/s13311-021-01048-z. Epub 2021 Apr 20. Neurotherapeutics. 2021. PMID: 33880738 Free PMC article. Review.
References
-
- Abu-Absi SF, Yang L, Thompson P, Jiang C, Kandula S, Schilling B, Shukla AA (2010) Defining process design space for monoclonal antibody cell culture. Biotechnol Bioeng 106:894–905 - PubMed
-
- Borys MC, Dalal NG, Abu-Absi NR, Khattak SF, Jing Y, Xing Z, Li ZJ (2010) Effects of culture conditions on N-glycolylneuraminic acid (Neu5Gc) content of a recombinant fusion protein produced in CHO cells. Biotechnol Bioeng 105:1048–1057 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

