Effects of chemically characterized fractions from aerial parts of Echinacea purpurea and E. angustifolia on myelopoiesis in rats
- PMID: 21870322
- PMCID: PMC3740717
- DOI: 10.1055/s-0031-1279990
Effects of chemically characterized fractions from aerial parts of Echinacea purpurea and E. angustifolia on myelopoiesis in rats
Abstract
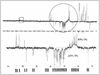
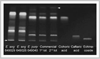
Echinacea species are used for beneficial effects on immune function, and various prevalent phytochemicals have immunomodulatory effects. Using a commercial E. purpurea (L.) Moench product, we have evaluated the myelopoietic effect on bone marrow of rats treated with various extracts and correlated this with their chemical class composition. Granulocyte/macrophage-colony forming cells (GM-CFCs) from femurs of female Sprague-Dawley rats were assessed at 24 h after 7 daily oral treatments. A 75% ethanolic extract at 50 mg dried weight (derived from 227 mg aerial parts) per kg body weight increased GM-CFCs by 70% but at 100 mg/kg was without effect. Ethanolic extracts from aerial parts of E. angustifolia DC. var. angustifolia and E. purpurea from the USDA North Central Regional Plant Introduction Station increased GM-CFCs by 3- and 2-fold, respectively, at 200 mg/kg (~1400 mg/kg plant material). Extract from another USDA E. angustifolia was inactive. Proton and APT NMR, MS, and TLC indicated alkylamides and caffeic-acid derivatives (CADs) present in ethanolic extracts of both the commercial and USDA-derived material. Cichoric and caftaric acids were prominent in both E. purpurea ethanolic extracts but absent in E. angustifolia. Aqueous extract of the commercial material exhibited polysaccharide and CAD signatures and was without effect on GM-CFCs. A methanol-CHCl3 fraction of commercial source, also inactive, was almost exclusively 1:4 nonanoic: decanoic acids, which were also abundant in commercial ethanolic extract but absent from USDA material. In conclusion, we have demonstrated an ethanolextractable myelostimulatory activity in Echinacea aerial parts that, when obtained from commercial herbal supplements, may be antagonized by medium-chain fatty acids presumably derived from a non-plant additive.
© Georg Thieme Verlag KG Stuttgart · New York.
Conflict of interest statement
Conflict of Interest
There are no conflicts of interest to disclose.
Figures




References
-
- Wills RBH, Bone K, Morgan M. Herbal products:active constituents, modes of action and quality control. Nutrition Res Rev. 2000;13:47–77. - PubMed
-
- Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench):a review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2005;57:929–954. - PubMed
-
- Gertsch J, Schoop R, Kuenzle U, Suter A. Echinacea alkylamides modulate TNF-alpha gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett. 2004;577:563–569. - PubMed
-
- Chen Y, Fu T, Tao T, Yang J, Chang Y, Wang M, Kim L, Qu L, Cassady J, Scalzo R, Wang X. Macrophage activating effects of new alkamides from the roots of Echinacea species. J Nat Prod. 2005;68:773–776. - PubMed
-
- Matthias A, Banbury L, Stevenson LM, Bone KM, Leach DN, Lehmann RP. Alkylamides from Echinacea modulate induced immune responses in macrophages. Immunol Invest. 2007;36:117–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

