Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders
- PMID: 21871372
- PMCID: PMC3164797
- DOI: 10.1016/j.jaac.2011.06.010
Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders
Abstract
Objective: To evaluate the efficacy and safety of osmotic-release methylphenidate (OROS-MPH) compared with placebo for attention-deficit/hyperactivity disorder (ADHD), and the impact on substance treatment outcomes in adolescents concurrently receiving cognitive-behavioral therapy (CBT) for substance use disorders (SUD).
Method: This was a 16-week, randomized, controlled, multi-site trial of OROS-MPH + CBT versus placebo + CBT in 303 adolescents (aged 13 through 18 years) meeting DSM-IV diagnostic criteria for ADHD and SUD. Primary outcome measures included the following: for ADHD, clinician-administered ADHD Rating Scale (ADHD-RS), adolescent informant; for substance use, adolescent-reported days of use in the past 28 days. Secondary outcome measures included parent ADHD-RS and weekly urine drug screens (UDS).
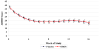
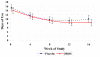
Results: There were no group differences on reduction in ADHD-RS scores (OROS-MPH: -19.2, 95% confidence interval [CI], -17.1 to -21.2; placebo, -21.2, 95% CI, -19.1 to -23.2) or reduction in days of substance use (OROS-MPH: -5.7 days, 95% CI, 4.0-7.4; placebo: -5.2 days, 95% CI, 3.5-7.0). Some secondary outcomes favored OROS-MPH, including lower parent ADHD-RS scores at 8 (mean difference = 4.4, 95% CI, 0.8-7.9) and 16 weeks (mean difference =6.9; 95% CI, 2.9-10.9) and more negative UDS in OROS-MPH (mean = 3.8) compared with placebo (mean = 2.8; p = .04).
Conclusions: OROS-MPH did not show greater efficacy than placebo for ADHD or on reduction in substance use in adolescents concurrently receiving individual CBT for co-occurring SUD. However, OROS-MPH was relatively well tolerated and was associated with modestly greater clinical improvement on some secondary ADHD and substance outcome measures. Clinical Trial Registration Information-Attention Deficit Hyperactivity Disorder (ADHD) in Adolescents with Substance Use Disorders (SUD); http://www.clinicaltrials.gov; NCT00264797.
Copyright © 2011 American Academy of Child and Adolescent Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Wilens TE, Gignac M, Swezey A, Monuteaux MC, Biederman J. Characteristics of adolescents and young adults with ADHD who divert or misuse their prescribed medications. J Am Acad Child Adolesc Psychiatry. 2006 Apr;45(4):408–414. - PubMed
-
- Kessler RC, Adler L, Ames M, et al. The prevalence and effects of adult attention deficit/hyperactivity disorder on work performance in a nationally representative sample of workers. J Occup Environ Med. 2005 Jun;47(6):565–572. - PubMed
-
- Katusic SK, Barbaresi WJ, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol. 2005 Oct;15(5):764–776. - PubMed
-
- Whitmore EA, Riggs PD. Developmentally informed diagnostic and treatment considerations in comorbid conditions. In: Liddle HA, Rowe CL, editors. Adolescent Substance Abuse: Research and Clinical Advances. Cambridge University Press; Cambridge, U.K.: 2006. pp. 264–283.
-
- Rowe CL, Liddle HA, Greenbaum PE, Henderson CE. Impact of psychiatric comorbidity on treatment of adolescent drug abusers. J Subst Abuse Treat. 2004 Mar;26(2):129–140. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 DA022284/DA/NIDA NIH HHS/United States
- U10 DA13034/DA/NIDA NIH HHS/United States
- U10 DA020036/DA/NIDA NIH HHS/United States
- U10 DA20024/DA/NIDA NIH HHS/United States
- U10 DA13043/DA/NIDA NIH HHS/United States
- K12 DA000357/DA/NIDA NIH HHS/United States
- U10 DA20036/DA/NIDA NIH HHS/United States
- U10 DA13732/DA/NIDA NIH HHS/United States
- U10 DA013034/DA/NIDA NIH HHS/United States
- U10 DA013727/DA/NIDA NIH HHS/United States
- U10 DA013720/DA/NIDA NIH HHS/United States
- U10 DA015831/DA/NIDA NIH HHS/United States
- U10 DA013035/DA/NIDA NIH HHS/United States
- U10 DA13727/DA/NIDA NIH HHS/United States
- U10 DA15831/DA/NIDA NIH HHS/United States
- U10 DA020024/DA/NIDA NIH HHS/United States
- U10 DA013732/DA/NIDA NIH HHS/United States
- U10 DA013043/DA/NIDA NIH HHS/United States
- U10 DA13716/DA/NIDA NIH HHS/United States
- U10 DA13720/DA/NIDA NIH HHS/United States
- K24 DA022412/DA/NIDA NIH HHS/United States
- U10 DA13035/DA/NIDA NIH HHS/United States
- U10 DA013716/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

