Liver toxicity and carcinogenicity in F344/N rats and B6C3F1 mice exposed to Kava Kava
- PMID: 21871523
- PMCID: PMC3190036
- DOI: 10.1016/j.fct.2011.07.067
Liver toxicity and carcinogenicity in F344/N rats and B6C3F1 mice exposed to Kava Kava
Abstract
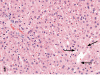
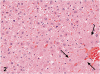
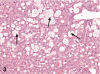
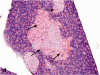
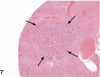
Kava Kava is an herbal supplement used as an alternative to antianxiety drugs. Although some reports suggest an association of Kava Kava with hepatotoxicity , it continues to be used in the United States due to lack of toxicity characterization. In these studies F344/N rats and B6C3F1 mice were administered Kava Kava extract orally by gavage in corn oil for two weeks, thirteen weeks or two years. Results from prechronic studies administered Kava Kava at 0.125 to 2g/kg body weight revealed dose-related increases in liver weights and incidences of hepatocellular hypertrophy. In the chronic studies, there were dose-related increases in the incidences of hepatocellular hypertrophy in rats and mice administered Kava Kava for up to 1g/kg body weight. This was accompanied by significant increases in incidences of centrilobular fatty change. There was no treatment- related increase in carcinogenic activity in the livers of male or female rats in the chronic studies. Male mice showed a significant dose-related increase in the incidence of hepatoblastomas. In female mice, there was a significant increase in the combined incidence of hepatocellular adenoma and carcinoma in the low and mid dose groups but not in the high dose group. These findings were accompanied by several nonneoplastic hepatic lesions.
Published by Elsevier Ltd.
Figures



References
-
- ABC News The Kava Craze: The Herb that May Relieve Stress and Anxiety. More Information on Kava. 1998 20/20 Airdate, June 22, 1998. < http://www.herbsnmore.com/2020rpt.html>.
-
- Allen DG, Pearse G, Haseman JK, Maronpot RR. Prediction of rodent carcinogenesis: An evaluation of prechronic liver lesions as forecasters of liver tumors in NTP carcinogenicity studies. Toxicol. Pathol. 2004;32:393–401. - PubMed
-
- Anke J, Ramzan I. Kava hepatotoxicity: Are we any closer to the truth? Planta Med. 2004;70:193–196. - PubMed
-
- Bailer AJ, Portier CJ. Effects of treatment-induced mortality and tumor-induced mortality on tests for carcinogenicity in small samples. Biometrics. 1988;44:417–431. - PubMed
-
- Basch E, Ulbricht C, Hammerness P, Tsouronis C, Sollars D, Rogers A, Basch S, Bent S, Boon H, Ernst E. Kava monograph: A clinical decision support tool. J. Herb Pharmacother. 2002;2:65–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

