Assessment of corneal thickness and keratocyte density in a rabbit model of laser in situ keratomileusis using scanning laser confocal microscopy
- PMID: 21871603
- PMCID: PMC3304505
- DOI: 10.1016/j.ajo.2011.05.023
Assessment of corneal thickness and keratocyte density in a rabbit model of laser in situ keratomileusis using scanning laser confocal microscopy
Abstract
Purpose: To determine the repeatability of corneal thickness and keratocyte density using in vivo confocal scanning laser microscopy in a rabbit model of laser in situ keratomileusis.
Design: Prospective, experimental animal study.
Methods: En face tomographic images of corneal tissue were captured from 5 New Zealand white rabbits. Central corneal thickness was compared with conventional ultrasonic pachymetry. Keratocyte density was measured as a function of stromal depth at baseline and 6 weeks after a 130-μm lamellar incision in the following regions: first countable stromal image (30 to 39 μm), anterior stroma (40 to 75 μm), incision zone (76 to 150 μm), mid stroma (151 to 250 μm), and deep stroma (251 to 400 μm).
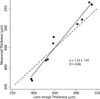
Results: The mean residual difference between ultrasonic and confocal corneal thickness measurements was 2.1 μm (95% confidence interval [CI], -7.0 to 11.2 μm; P = .61). Before the lamellar incision, keratocyte density was highest in the first countable frame of the anterior stroma, 53 800 cells/mm(3) (95% CI, 35 000 to 72 000 cells/mm(3)) and was least in deep stroma, 27 100 cells/mm(3) (95% CI, 22 400 to 32 000 cells/mm(3)). Six weeks after stromal lamellar incision, keratocyte density was unchanged in the first countable frame of the anterior stroma, 43 700 cells/mm(3) (95% CI, 31 800 to 55 500 cells/mm(3); P = .29). There were no changes in cell density in deeper stromal regions.
Conclusions: There was excellent agreement between ultrasonic and confocal microscopy measurements of corneal thickness. In vivo repeatability of keratocyte density estimation using scanning laser confocal microscopy is comparable with the results of previous reports using tandem-scanning confocal microscopy. Keratocyte density was more varied, but not significantly different, in the anterior-most corneal stroma 6 weeks after a lamellar incision.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


















References
-
- Honda N, Miyai T, Nejima R, et al. Effect of latanoprost on the expression of matrix metalloproteinases and tissue inhibitor of metalloproteinase 1 on the ocular surface. Arch Ophthalmol. 2010;128(4):466–471. - PubMed
-
- Erie JC, Patel SV, McLaren JW, Hodge DO, Bourne WM. Corneal keratocyte deficits after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 2006;141(5):799–809. - PubMed
-
- Knappe S, Stachs O, Zhivov A, Hovakimyan M, Guthoff R. Results of confocal microscopy examinations after collagen cross-linking with riboflavin and uva light in patients with progressive keratoconus. Ophthalmologica. 2011;225(2):95–104. - PubMed
-
- Hovakimyan M, Guthoff R, Knappe S, et al. Short-term corneal response to cross-linking in rabbit eyes assessed by in vivo confocal laser scanning microscopy and histology. Cornea. 2011;30(2):196–203. - PubMed
-
- de Benito Llopis L, Drake P, Canadas P, Hernandez-Verdejo JL, Teus MA. Keratocyte density after laser-assisted subepithelial keratectomy with mitomycin c. Am J Ophthalmol. 2010;150(5):642–649. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

