An overview of enzymatic reagents for the removal of affinity tags
- PMID: 21871965
- PMCID: PMC3195948
- DOI: 10.1016/j.pep.2011.08.005
An overview of enzymatic reagents for the removal of affinity tags
Abstract
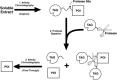
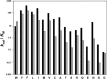
Although they are often exploited to facilitate the expression and purification of recombinant proteins, every affinity tag, whether large or small, has the potential to interfere with the structure and function of its fusion partner. For this reason, reliable methods for removing affinity tags are needed. Only enzymes have the requisite specificity to be generally useful reagents for this purpose. In this review, the advantages and disadvantages of some commonly used endo- and exoproteases are discussed in light of the latest information.
Published by Elsevier Inc.
Figures


References
-
- Schechter I., Berger A. On the size of the active site in proteases. Biochem. Biophys. Res. Com. 1967;27:157–162. - PubMed
-
- Waugh D.S. Making the most of affinity tags. Trends Biotechnol. 2005;23:316–320. - PubMed
-
- Arnau J., Lauritzen C., Petersen G.E., Pedersen J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr. Purif. 2006;48:1–13. - PubMed
-
- Sun-Lailam B.B., Hevel J.M. Efficient cleavage of problematic tobacco etch virus (TEV)-protein arginine methyltransferase constructs. Anal. Biochem. 2009;387:130–132. - PubMed
-
- Wu J., Filutowicz M. Hexahistidine (His6)-tag dependent protein dimerization: a cautionary tale. Acta Biochim. Pol. 1999;46:591–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

