Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan
- PMID: 21872361
- PMCID: PMC3227765
- DOI: 10.1016/j.neurobiolaging.2011.07.006
Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan
Abstract
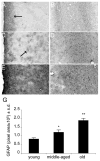
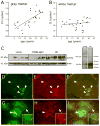
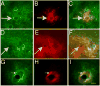
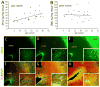
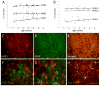
The glycosaminoglycan hyaluronan (HA) accumulates in central nervous system lesions where it limits astrogliosis but also inhibits oligodendrocyte progenitor cell (OPC) maturation. The role of hyaluronan in normative brain aging has not been previously investigated. Here, we tested the hypothesis that HA accumulates in the aging nonhuman primate brain. We found that HA levels significantly increase with age in the gray matter of rhesus macaques. HA accumulation was linked to age-related increases in the transcription of HA synthase-1 (HAS1) expressed by reactive astrocytes but not changes in the expression of other HAS genes or hyaluronidases. HA accumulation was accompanied by increased expression of CD44, a transmembrane HA receptor. Areas of gray matter with elevated HA in older animals demonstrated increased numbers of olig2(+) OPCs, consistent with the notion that HA may influence OPC expansion or maturation. Collectively, these data indicate that HAS1 and CD44 are transcriptionally upregulated in astrocytes during normative aging and are linked to HA accumulation in gray matter.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Akiyama H, Tooyama I, Kawamata T, Ikeda K, McGeer PL. Morphological diversities of CD44 positive astrocytes in the cerebral cortex of normal subjects and patients with Alzheimer’s disease. Brain Res. 1993;632(1–2):249–59. - PubMed
-
- Alldinger S, Fonfara S, Kremmer E, Baumgartner W. Up-regulation of the hyaluronate receptor CD44 in canine distemper demyelinated plaques. Acta Neuropathol (Berl) 2000;99(2):138–46. - PubMed
-
- Asher R, Bignami A. Hyaluronate binding and CD44 expression in human glioblastoma cells and astrocytes. Exp Cell Res. 1992;203(1):80–90. - PubMed
-
- Asher R, Perides G, Vanderhaeghen JJ, Bignami A. Extracellular matrix of central nervous system white matter: demonstration of an hyaluronate-protein complex. J Neurosci Res. 1991;28(3):410–21. - PubMed
-
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11(9):966–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

