Identification and characterization of cartilage oligomeric matrix protein as a novel pathogenic factor in keloids
- PMID: 21872564
- PMCID: PMC3181360
- DOI: 10.1016/j.ajpath.2011.06.034
Identification and characterization of cartilage oligomeric matrix protein as a novel pathogenic factor in keloids
Abstract
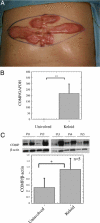
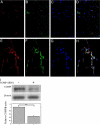
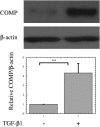
To elucidate pathogenic molecules in keloids, microarray analysis was performed using RNAs extracted from keloid-derived fibroblasts and normal skin-derived fibroblasts from the same patient with a typical keloid. Among 11 up-regulated extracellular matrix genes, cartilage oligomeric matrix protein (COMP) was most prominently increased. Up-regulation of COMP mRNA and protein was confirmed in the keloid tissue by quantitative RT-PCR and Western blot. Using immunohistochemistry, we compared 15 keloids and 6 control normal tissues using a COMP-specific antibody and found that COMP stained positively in 10 keloids (66.7%), whereas no staining was observed in normal tissues, demonstrating the ectopic expression of COMP in keloids. Comparing keloids smaller or larger than 10 cm(2), the larger keloids were significantly more intensely stained with the COMP-specific antibody. Because COMP reportedly accelerates collagen type I fibril assembly, we examined whether extracellular type I collagen deposition is altered by silencing COMP mRNA by small interfering RNA (siRNA). Immunocytochemistry showed at 96 hours after transfection with COMP siRNA that the extracellular deposition of type I collagen was decreased compared to that observed with control siRNA. Further, COMP knockdown decreased amount collagens type I to V in the medium and on the cell surfaces. Our data suggest that COMP facilitates keloid formation by accelerating collagen deposition, thus providing a new therapeutic target.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Treatment of keloids through Runx2 siRNA‑induced inhibition of the PI3K/AKT signaling pathway.Mol Med Rep. 2021 Jan;23(1):55. doi: 10.3892/mmr.2020.11693. Epub 2020 Nov 17. Mol Med Rep. 2021. PMID: 33200804 Free PMC article.
-
Cartilage oligomeric matrix protein is overexpressed by scleroderma dermal fibroblasts.Matrix Biol. 2006 May;25(4):213-22. doi: 10.1016/j.matbio.2006.01.007. Epub 2006 Mar 7. Matrix Biol. 2006. PMID: 16520029
-
Knockdown of elF3a inhibits TGF‑β1‑induced extracellular matrix protein expression in keloid fibroblasts.Mol Med Rep. 2018 Mar;17(3):4057-4061. doi: 10.3892/mmr.2017.8365. Epub 2017 Dec 29. Mol Med Rep. 2018. PMID: 29286129
-
Keloid disorder: Fibroblast differentiation and gene expression profile in fibrotic skin diseases.Exp Dermatol. 2021 Jan;30(1):132-145. doi: 10.1111/exd.14243. Epub 2020 Dec 20. Exp Dermatol. 2021. PMID: 33211348 Review.
-
The Communication from Immune Cells to the Fibroblasts in Keloids: Implications for Immunotherapy.Int J Mol Sci. 2023 Oct 23;24(20):15475. doi: 10.3390/ijms242015475. Int J Mol Sci. 2023. PMID: 37895153 Free PMC article. Review.
Cited by
-
Abnormal collagen deposition mediated by cartilage oligomeric matrix protein in the pathogenesis of oral submucous fibrosis.Int J Oral Sci. 2025 Mar 27;17(1):25. doi: 10.1038/s41368-025-00355-x. Int J Oral Sci. 2025. PMID: 40148275 Free PMC article.
-
Site-specific gene expression profiling as a novel strategy for unravelling keloid disease pathobiology.PLoS One. 2017 Mar 3;12(3):e0172955. doi: 10.1371/journal.pone.0172955. eCollection 2017. PLoS One. 2017. PMID: 28257480 Free PMC article.
-
Scleroderma with Nodular Scleroderma.Case Rep Dermatol. 2016 Nov 14;8(3):303-310. doi: 10.1159/000452324. eCollection 2016 Sep-Dec. Case Rep Dermatol. 2016. PMID: 27920682 Free PMC article.
-
Cartilage oligomeric matrix protein participates in the pathogenesis of liver fibrosis.J Hepatol. 2016 Nov;65(5):963-971. doi: 10.1016/j.jhep.2016.06.003. Epub 2016 Jun 15. J Hepatol. 2016. PMID: 27318326 Free PMC article.
-
Epidermal Growth Factor (EGF)-Like Repeats and Discoidin I-Like Domains 3 (EDIL3): A Potential New Therapeutic Tool for the Treatment of Keloid Scars.Tissue Eng Regen Med. 2017 Apr 7;14(3):267-277. doi: 10.1007/s13770-017-0034-5. eCollection 2017 Jun. Tissue Eng Regen Med. 2017. PMID: 30603483 Free PMC article.
References
-
- Brown B.C., McKenna S.P., Siddhi K., McGrouther D.A., Bayat A. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg. 2008;61:1049–1058. - PubMed
-
- Shih B., Garside E., McGrouther D.A., Bayat A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010;18:139–153. - PubMed
-
- Russell J.D., Witt W.S. Cell size and growth characteristics of cultured fibroblasts isolated from normal and keloid tissue. Plast Reconstr Surg. 1976;57:207–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

