Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism
- PMID: 21872687
- PMCID: PMC3261372
- DOI: 10.1016/j.bone.2011.08.010
Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism
Abstract
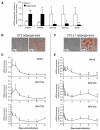
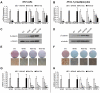
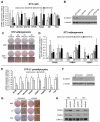
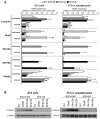
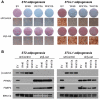
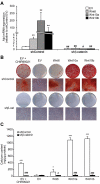
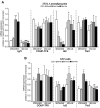
Wnt10b is an established regulator of mesenchymal stem cell (MSC) fate that inhibits adipogenesis and stimulates osteoblastogenesis, thereby impacting bone mass in vivo. However, downstream mechanisms through which Wnt10b exerts these effects are poorly understood. Moreover, whether other endogenous Wnt ligands also modulate MSC fate remains to be fully addressed. In this study, we identify Wnt6 and Wnt10a as additional Wnt family members that, like Wnt10b, are downregulated during development of white adipocytes in vivo and in vitro, suggesting that Wnt6 and/or Wnt10a may also inhibit adipogenesis. To assess the relative activities of Wnt6, Wnt10a and Wnt10b to regulate mesenchymal cell fate, we used gain- and loss-of function approaches in bipotential ST2 cells and in 3T3-L1 preadipocytes. Enforced expression of Wnt10a stabilizes β-catenin, suppresses adipogenesis and stimulates osteoblastogenesis to a similar extent as Wnt10b, whereas stable expression of Wnt6 has a weaker effect on these processes than Wnt10a or Wnt10b. In contrast, knockdown of endogenous Wnt6 is associated with greater preadipocyte differentiation and impaired osteoblastogenesis than knockdown of Wnt10a or Wnt10b, suggesting that, among these Wnt ligands, Wnt6 is the most potent endogenous regulator of MSC fate. Finally, we show that knockdown of β-catenin completely prevents the inhibition of adipogenesis and stimulation of osteoblast differentiation by Wnt6, Wnt10a or Wnt10b. Potential mechanisms whereby Wnts regulate fate of MSCs downstream of β-catenin are also investigated. In conclusion, this study identifies Wnt10a and Wnt6 as additional regulators of MSC fate and demonstrates that mechanisms downstream of β-catenin are required for Wnt6, Wnt10a and Wnt10b to influence differentiation of mesenchymal precursors.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
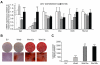

References
-
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–3. l. - PubMed
-
- Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–1004. l. - PubMed
-
- Wright WS, Longo KA, Dolinsky VW, Gerin I, Kang S, Bennett CN, Chiang SH, Prestwich TC, Gress C, Burant CF, Susulic VS, MacDougald OA. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56:295–303. l. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

