Temporal and spatial correlation of platelet-activating factor-induced increases in endothelial [Ca²⁺]i, nitric oxide, and gap formation in intact venules
- PMID: 21873500
- PMCID: PMC3213973
- DOI: 10.1152/ajpheart.00599.2011
Temporal and spatial correlation of platelet-activating factor-induced increases in endothelial [Ca²⁺]i, nitric oxide, and gap formation in intact venules
Abstract
We have previously demonstrated that platelet-activating factor (PAF)-induced increases in microvessel permeability were associated with endothelial gap formation and that the magnitude of peak endothelial intracellular Ca(2+) concentration ([Ca(2+)](i)) and nitric oxide (NO) production at the single vessel level determines the degree of the permeability increase. This study aimed to examine whether the magnitudes of PAF-induced peak endothelial [Ca(2+)](i), NO production, and gap formation are correlated at the individual endothelial cell level in intact rat mesenteric venules. Endothelial gaps were quantified by the accumulation of fluorescent microspheres at endothelial clefts using confocal imaging. Endothelial [Ca(2+)](i) was measured on fura-2- or fluo-4-loaded vessels, and 4,5-diaminofluorescein (DAF-2) was used for NO measurements. The results showed that increases in endothelial [Ca(2+)](i), NO production, and gap formation occurred in all endothelial cells when vessels were exposed to PAF but manifested a spatial heterogeneity in magnitudes among cells in each vessel. PAF-induced peak endothelial [Ca(2+)](i) preceded the peak NO production by 0.6 min at the cellular level, and the magnitudes of NO production and gap formation linearly correlated with that of the peak endothelial [Ca(2+)](i) in each cell, suggesting that the initial levels of endothelial [Ca(2+)](i) determine downstream NO production and gap formation. These results provide direct evidence from intact venules that inflammatory mediator-induced increases in microvessel permeability are associated with the generalized formation of endothelial gaps around all endothelial cells. The spatial differences in the molecular signaling that were initiated by the heterogeneous endothelial Ca(2+) response contribute to the heterogeneity in permeability increases along the microvessel wall during inflammation.
Figures



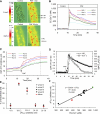
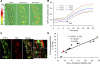
References
-
- Adamson RH, Zeng M, Adamson GN, Lenz JF, Curry FE. PAF- and bradykinin-induced hyperpermeability of rat venules is independent of actin-myosin contraction. Am J Physiol Heart Circ Physiol 285: H406–H417, 2003 - PubMed
-
- Baldwin AL. Modified hemoglobins produce venular interendothelial gaps and albumin leakage in the rat mesentery. Am J Physiol Heart Circ Physiol 277: H650–H659, 1999 - PubMed
-
- Braverman IM, Keh-Yen A. Three-dimensional reconstruction of endothelial cell gaps in psoriatic vessels and their morphologic identity with gaps produced by the intradermal injection of histamine. J Invest Dermatol 86: 577–581, 1986 - PubMed
-
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6: 1362–1367, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

