Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice
- PMID: 21873652
- PMCID: PMC3199511
- DOI: 10.1074/jbc.M111.250449
Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice
Abstract
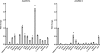
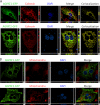
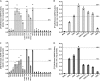
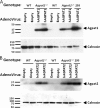
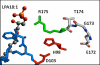
Loss-of-function mutations in 1-acylglycerol-3-phosphate O-acyltransferase (AGPAT) 2 in humans and mice result in loss of both the white and brown adipose tissues from birth. AGPAT2 generates precursors for the synthesis of glycerophospholipids and triacylglycerols. Loss of adipose tissue, or lipodystrophy, results in hyperinsulinemia, diabetes mellitus, and severe hepatic steatosis. Here, we analyzed biochemical properties of human AGPAT2 and its close homolog, AGPAT1, and we studied their role in liver by transducing their expression via recombinant adenoviruses in Agpat2(-/-) mice. The in vitro substrate specificities of AGPAT1 and AGPAT2 are quite similar for lysophosphatidic acid and acyl-CoA. Protein homology modeling of both the AGPATs with glycerol-3-phosphate acyltransferase 1 (GPAT1) revealed that they have similar tertiary protein structure, which is consistent with their similar substrate specificities. When co-expressed, both isoforms co-localize to the endoplasmic reticulum. Despite such similarities, restoring AGPAT activity in liver by overexpression of either AGPAT1 or AGPAT2 in Agpat2(-/-) mice failed to ameliorate the hepatic steatosis. From these studies, we suggest that the role of AGPAT1 or AGPAT2 in liver lipogenesis is minimal and that accumulation of liver fat is primarily a consequence of insulin resistance and loss of adipose tissue in Agpat2(-/-) mice.
Figures


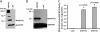



References
-
- McMaster C. R., Jackson T. R. (2004) in Lipid Metabolism and Membrane Biogenesis (Daum G. ed) pp. 5–30, Springer-Verlag, Berlin
-
- Coleman R. A., Lee D. P. (2004) Prog. Lipid Res. 43, 134–176 - PubMed
-
- Leung D. W. (2001) Front. Biosci. 6, D944–D953 - PubMed
-
- Agarwal A. K., Garg A. (2003) Trends Endocrinol. Metab. 14, 214–221 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

