Quantification of peripapillary sparing and macular involvement in Stargardt disease (STGD1)
- PMID: 21873672
- PMCID: PMC3220414
- DOI: 10.1167/iovs.11-7693
Quantification of peripapillary sparing and macular involvement in Stargardt disease (STGD1)
Abstract
Purpose: To quantify and compare structure and function across the macula and peripapillary area in Stargardt disease (STGD1).
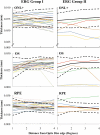
Methods: Twenty-seven patients (27 eyes) and 12 age-similar controls (12 eyes) were studied. Patients were classified on the basis of full-field electroretinogram (ERG) results: Fundus autofluorescence (FAF) and spectral domain-optical coherence tomography (SD-OCT) horizontal line scans were obtained through the fovea and peripapillary area. The thicknesses of the outer nuclear layer plus outer plexiform layer (ONL+), outer segment (OS), and retinal pigment epithelium (RPE) were measured through the fovea, and peripapillary areas from 1° to 4° temporal to the optic disc edge using a computer-aided, manual segmentation technique. Visual sensitivities in the central 10° were assessed using microperimetry and related to retinal layer thicknesses.
Results: Compared to the central macula, the differences between controls and patients in ONL+, OS, and RPE layer thicknesses were less in the nasal and temporal macula. Relative sparing of the ONL+ and/or OS layers was detected in the nasal (i.e., peripapillary) macula in 8 of 13 patients with extramacular disease on FAF; relative functional sparing was also detected in this subgroup. All 14 patients with disease confined to the central macula, as detected on FAF, showed ONL+ and OS layer thinning in regions of normal RPE thickness.
Conclusions: Relative peripapillary sparing was detected in STGD1 patients with extramacular disease on FAF. Photoreceptor thinning may precede RPE degeneration in STGD1.
Figures






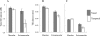
References
-
- Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt's macular dystrophy. Nat Genet. 1997;15:236–246 - PubMed
-
- Schwoerer J, Secrétan M, Zografos L, Piguet B. Indocyanine green angiography in fundus flavimaculatus. Ophthalmologica. 2000;214:240–245 - PubMed
-
- Klein R, Lewis RA, Meyers SM, Myers FL. Subretinal neovascularization associated with fundus flavimaculatus. Arch Ophthalmol. 1978;96:2054–2057 - PubMed
-
- Lois N, Halfyard AS, Bird AC, Holder GE, Fitzke FW. Fundus autofluorescence in Stargardt macular dystrophy-fundus flavimaculatus. Am J Ophthalmol. 2004;138:55–63 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

