A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories
- PMID: 21873981
- PMCID: PMC3209781
- DOI: 10.1038/emboj.2011.293
A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories
Abstract
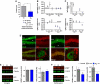
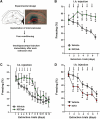
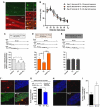
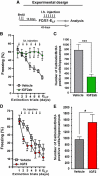
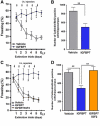
Extinction learning refers to the phenomenon that a previously learned response to an environmental stimulus, for example, the expression of an aversive behaviour upon exposure to a specific context, is reduced when the stimulus is repeatedly presented in the absence of a previously paired aversive event. Extinction of fear memories has been implicated with the treatment of anxiety disease but the molecular processes that underlie fear extinction are only beginning to emerge. Here, we show that fear extinction initiates upregulation of hippocampal insulin-growth factor 2 (Igf2) and downregulation of insulin-growth factor binding protein 7 (Igfbp7). In line with this observation, we demonstrate that IGF2 facilitates fear extinction, while IGFBP7 impairs fear extinction in an IGF2-dependent manner. Furthermore, we identify one cellular substrate of altered IGF2 signalling during fear extinction. To this end, we show that fear extinction-induced IGF2/IGFBP7 signalling promotes the survival of 17-19-day-old newborn hippocampal neurons. In conclusion, our data suggest that therapeutic strategies that enhance IGF2 signalling and adult neurogenesis might be suitable to treat disease linked to excessive fear memory.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

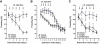
References
-
- Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS (2003) IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci 24: 23–40 - PubMed
-
- Aimone JB, Wiles J, Gage FH (2006) Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci 9: 723–727 - PubMed
-
- Alexa A, Rahnenfuhrer J, Lengauer T (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

