Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation
- PMID: 21874295
- PMCID: PMC3535207
- DOI: 10.1007/s00586-011-1976-2
Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation
Abstract
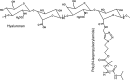
Introduction: Thermoreversible hydrogels have potential in spine research as they provide easy injectability and mild gelling mechanism (by physical cross-link). The purpose of this study was to assess the potential of thermoreversible hyaluronan-based hydrogels (HA-pNIPAM) (pNIPAM Mn = 10, 20, 35 × 10(3) g mol(-1)) as nucleus pulposus cells (NPC) carrier.
Materials and methods: Cytocompatibility (WST-1 assay), viability (trypan blue), morphology (toluidine blue), sulphated glycosaminoglycan synthesis (DMMB assay) and gene expression profile (real-time PCR) of bovine NPC cultured in HA-pNIPAM were followed for 1 week and compared to alginate gel bead cultures. The injectability and cell survival in a whole disc organ culture model were assessed up to day 7.
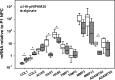
Results: All HA, HA-pNIPAM and their degradation products were cytocompatible to NPC. HA-pNIPAM hydrogels with no volume change upon gelling maintained NPC viability and characteristic rounded morphology. Glycosaminoglycan synthesis was similar in HA-pNIPAM and alginate gels. Following NPC expansion, both gels induced re-differentiation toward the NPC phenotype. Significant differences between the two gels were found for COLI, COLII, HAS1, HAS2 and ADAMTS4 but not for MMPs and TIMPs. Higher expression of hyaluronan synthases (HAS1, HAS2) and lower expression of COLI and COLII mRNA were noted in cells cultured in HA-pNIPAM (pNIPAM = 20 × 10(3)g mol(-1)). NPC suspension in HA-pNIPAM was injectable through a 22-G needle without loss of cell viability. Ex vivo, NPC viability was maintained in HA-pNIPAM for 1 week.
Conclusion: A HA-pNIPAM composition suitable for nucleus pulposus repair that provides an injectable carrier for NPC, maintains their phenotype and promotes extracellular matrix generation was identified.
Figures







Similar articles
-
Thermoreversible hyaluronan-based hydrogel supports in vitro and ex vivo disc-like differentiation of human mesenchymal stem cells.Spine J. 2013 Nov;13(11):1627-39. doi: 10.1016/j.spinee.2013.05.029. Epub 2013 Jul 3. Spine J. 2013. PMID: 23830827
-
Biomimetic fibrin-hyaluronan hydrogels for nucleus pulposus regeneration.Regen Med. 2014 May;9(3):309-26. doi: 10.2217/rme.14.5. Regen Med. 2014. PMID: 24935043
-
Chondroprotective supplementation promotes the mechanical properties of injectable scaffold for human nucleus pulposus tissue engineering.J Mech Behav Biomed Mater. 2014 Jan;29:56-67. doi: 10.1016/j.jmbbm.2013.08.020. Epub 2013 Aug 28. J Mech Behav Biomed Mater. 2014. PMID: 24055794
-
A Hyaluronan and Platelet-Rich Plasma Hydrogel for Mesenchymal Stem Cell Delivery in the Intervertebral Disc: An Organ Culture Study.Int J Mol Sci. 2021 Mar 15;22(6):2963. doi: 10.3390/ijms22062963. Int J Mol Sci. 2021. PMID: 33803999 Free PMC article.
-
Delivery of EPC embedded in HA-hydrogels for treatment of acute kidney injury.Biomatter. 2013 Jan-Mar;3(1):e23284. doi: 10.4161/biom.23284. Epub 2013 Jan 1. Biomatter. 2013. PMID: 23507925 Free PMC article. Review.
Cited by
-
[Research progress of intervertebral disc endogenous stem cells for intervertebral disc regeneration].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017 Oct 15;31(10):1267-1272. doi: 10.7507/1002-1892.201703036. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017. PMID: 29806333 Free PMC article. Chinese.
-
Self-healing injectable multifunctional hydrogels for intervertebral disc disease.Mater Today Bio. 2025 Mar 11;32:101655. doi: 10.1016/j.mtbio.2025.101655. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40166378 Free PMC article. Review.
-
Bio-engineered thermo-sensitive alginate/PNIA-g-CS co-polymeric injectable hydrogel laden with GDF-5 to stimulate nucleus pulposus for IVD regeneration.J Biol Eng. 2025 May 22;19(1):49. doi: 10.1186/s13036-025-00520-0. J Biol Eng. 2025. PMID: 40405200 Free PMC article.
-
Multiscale Regulation of the Intervertebral Disc: Achievements in Experimental, In Silico, and Regenerative Research.Int J Mol Sci. 2021 Jan 12;22(2):703. doi: 10.3390/ijms22020703. Int J Mol Sci. 2021. PMID: 33445782 Free PMC article. Review.
-
Extra Cellular Matrix Remodeling: An Adjunctive Target for Spinal Cord Injury and Intervertebral Disc Degeneration.Neurospine. 2022 Sep;19(3):632-645. doi: 10.14245/ns.2244366.183. Epub 2022 Sep 30. Neurospine. 2022. PMID: 36203290 Free PMC article.
References
-
- Schizas C, Kulik G, Kosmopoulos V. Disc degeneration: current surgical options. Eur Cell Mater. 2010;20:306–315. - PubMed
-
- Grad S, Alini M, Eglin D, Sakai D, Mochida J, Mahor S et al (2010) Cells and biomaterials for intervertebral disc regeneration. In: Athanasiou KA (ed) Synthesis lectures on tissue engineering, vol 2. Morgan & Claypool, San Rafael, pp 1–104
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

