Mitochondrial ATP synthase: architecture, function and pathology
- PMID: 21874297
- PMCID: PMC3278611
- DOI: 10.1007/s10545-011-9382-9
Mitochondrial ATP synthase: architecture, function and pathology
Abstract
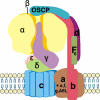
Human mitochondrial (mt) ATP synthase, or complex V consists of two functional domains: F(1), situated in the mitochondrial matrix, and F(o), located in the inner mitochondrial membrane. Complex V uses the energy created by the proton electrochemical gradient to phosphorylate ADP to ATP. This review covers the architecture, function and assembly of complex V. The role of complex V di-and oligomerization and its relation with mitochondrial morphology is discussed. Finally, pathology related to complex V deficiency and current therapeutic strategies are highlighted. Despite the huge progress in this research field over the past decades, questions remain to be answered regarding the structure of subunits, the function of the rotary nanomotor at a molecular level, and the human complex V assembly process. The elucidation of more nuclear genetic defects will guide physio(patho)logical studies, paving the way for future therapeutic interventions.
Figures
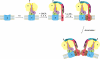
References
-
- Abu-Amero KK, Bosley TM. Mitochondrial abnormalities in patients with LHON-like optic neuropathies. Invest Ophthalmol Vis Sci. 2006;47:4211–4220. - PubMed
-
- Ackerman SH. Atp11p and Atp12p are chaperones for F(1)-ATPase biogenesis in mitochondria. Biochim Biophys Acta. 2002;1555:101–105. - PubMed
-
- Adachi K, Oiwa K, Nishizaka T, et al. Coupling of rotation and catalysis in F(1)-ATPase revealed by single-molecule imaging and manipulation. Cell. 2007;130:309–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

