Purification of the proprotein convertase furin by affinity chromatography based on PC-specific inhibitors
- PMID: 21875402
- PMCID: PMC3791315
- DOI: 10.1515/BC.2011.100
Purification of the proprotein convertase furin by affinity chromatography based on PC-specific inhibitors
Abstract
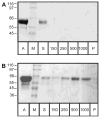
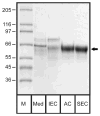
In eucaryotes, many secreted proteins and peptides are proteolytically excised from larger precursor proteins by a specific class of serine proteases, the proprotein/prohormone convertases (PCs). This cleavage is essential for substrate activation, making the PCs very interesting pharmacological targets in cancer and infectious disease research. Correspondingly, their structure, function and inhibition are intensely studied - studies that require the respective target proteins in large amounts and at high purity. Here we describe the development of a novel purification protocol of furin, the best-studied member of the PC family. We combined the heterologous expression of furin from CHO cells with a novel purification scheme employing an affinity step that efficiently extracts only active furin from the conditioned medium by using furin-specific inhibitor moieties as bait. Several potential affinity tags were synthesized and their binding to furin characterized. The best compound, Biotin-(Adoa)(2)-Arg-Pro-Arg-4-Amba coupled to streptavidin-Sepharose beads, was used in a three-step chromatographic protocol and routinely resulted in a high yield of a homogeneous furin preparation with a specific activity of ~60 units/mg protein. This purification and the general strategy can easily be adapted to the efficient purification of other PC family members.
Figures
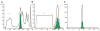

References
-
- Angliker H. Synthesis of tight binding inhibitors and their action on the proprotein-processing enzyme furin. J Med Chem. 1995;38:4014–4018. - PubMed
-
- Basak A. Inhibitors of proprotein convertases. J Mol Med. 2005;83:844–855. - PubMed
-
- Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol Carcinog. 2005;44:151–161. - PubMed
-
- Becker GL, Hardes K, Steinmetzer T. New substrate analogue furin inhibitors derived from 4-amidinobenzylamide. Bioorg Med Chem Lett. 2011;21:4695–4697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
