Protein folding and quality control in the ER
- PMID: 21875985
- PMCID: PMC3220362
- DOI: 10.1101/cshperspect.a007526
Protein folding and quality control in the ER
Erratum in
-
Protein folding and quality control in the ER.Cold Spring Harb Perspect Biol. 2012 Aug 1;4(8):a015438. doi: 10.1101/cshperspect.a015438. Cold Spring Harb Perspect Biol. 2012. PMID: 22855729 Free PMC article. Review. No abstract available.
Abstract
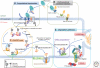
The endoplasmic reticulum (ER) uses an elaborate surveillance system called the ER quality control (ERQC) system. The ERQC facilitates folding and modification of secretory and membrane proteins and eliminates terminally misfolded polypeptides through ER-associated degradation (ERAD) or autophagic degradation. This mechanism of ER protein surveillance is closely linked to redox and calcium homeostasis in the ER, whose balance is presumed to be regulated by a specific cellular compartment. The potential to modulate proteostasis and metabolism with chemical compounds or targeted siRNAs may offer an ideal option for the treatment of disease.
Figures


References
-
- Aebi M, Bernasconi R, Clerc S, Molinari M 2010. N-glycan structures: Recognition and processing in the ER. Trends Biochem Sci 35: 74–82 - PubMed
-
- Appenzeller-Herzog C 2011. Glutathione- and non-glutathione-based oxidant control in the endoplasmic reticulum. J Cell Sci 124: 847–855 - PubMed
-
- Appenzeller-Herzog C, Ellgaard L 2008. The human PDI family: Versatility packed into a single fold. Biochim Biophys Acta 1783: 535–548 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
