Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula
- PMID: 21876058
- PMCID: PMC3195049
- DOI: 10.1128/AAC.05028-11
Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula
Abstract
The use of combination antibiotic therapy may be beneficial against rapidly emerging resistance in Pseudomonas aeruginosa. The aim of this study was to systematically investigate in vitro bacterial killing and resistance emergence with colistin alone and in combination with imipenem against multidrug-resistant (MDR) P. aeruginosa. Time-kill studies were conducted over 48 h using 5 clinical isolates and ATCC 27853 at two inocula (~10(6) and ~10(8) CFU/ml); MDR, non-MDR, and colistin-heteroresistant and -resistant strains were included. Nine colistin-imipenem combinations were investigated. Microbiological response was examined by log changes at 6, 24, and 48 h. Colistin combined with imipenem at clinically relevant concentrations increased the levels of killing of MDR and colistin-heteroresistant isolates at both inocula. Substantial improvements in activity with combinations were observed across 48 h with all colistin concentrations at the low inoculum and with colistin at 4× and 16× MIC (or 4 and 32 mg/liter) at the high inoculum. Combinations were additive or synergistic against imipenem-resistant isolates (MICs, 16 and 32 mg/liter) at the 10(6)-CFU inoculum in 9, 11, and 12 of 18 cases (i.e., 9 combinations across 2 isolates) at 6, 24, and 48 h, respectively, and against the same isolates at the 10(8)-CFU inoculum in 11, 7, and 8 cases, respectively. Against a colistin-resistant strain (MIC, 128 mg/liter), combinations were additive or synergistic in 9 and 8 of 9 cases at 24 h at the 10(6)- and 10(8)-CFU inocula, respectively, and in 5 and 7 cases at 48 h. This systematic study provides important information for optimization of colistin-imipenem combinations targeting both colistin-susceptible and colistin-resistant subpopulations.
Figures
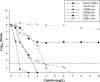


References
-
- Antoniadou A., et al. 2007. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J. Antimicrob. Chemother. 59:786–790 - PubMed
-
- Aoki N., et al. 2009. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother. 63:534–542 - PubMed
-
- Bergen P. J., et al. 2008. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 61:636–642 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

