Differential radial capillary action of ligand assay for high-throughput detection of protein-metabolite interactions
- PMID: 21876132
- PMCID: PMC3174574
- DOI: 10.1073/pnas.1018949108
Differential radial capillary action of ligand assay for high-throughput detection of protein-metabolite interactions
Abstract
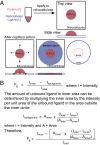
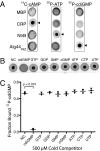
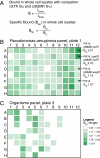
Interactions of proteins with low-molecular-weight ligands, such as metabolites, cofactors, and allosteric regulators, are important determinants of metabolism, gene regulation, and cellular homeostasis. Pharmaceuticals often target these interactions to interfere with regulatory pathways. We have developed a rapid, precise, and high-throughput method for quantitatively measuring protein-ligand interactions without the need to purify the protein when performed in cells with low background activity. This method, differential radial capillary action of ligand assay (DRaCALA), is based on the ability of dry nitrocellulose to separate the free ligand from bound protein-ligand complexes. Nitrocellulose sequesters proteins and bound ligand at the site of application, whereas free ligand is mobilized by bulk movement of the solvent through capillary action. We show here that DRaCALA allows detection of specific interactions between three nucleotides and their cognate binding proteins. DRaCALA allows quantitative measurement of the dissociation constant and the dissociation rate. Furthermore, DRaCALA can detect the expression of a cyclic-di-GMP (cdiGMP)-binding protein in whole-cell lysates of Escherichia coli, demonstrating the power of the method to bypass the prerequisite for protein purification. We have used DRaCALA to investigate cdiGMP signaling in 54 bacterial species from 37 genera and 7 eukaryotic species. These studies revealed the presence of potential cdiGMP-binding proteins in 21 species of bacteria, including 4 unsequenced species. The ease of obtaining metabolite-protein interaction data using the DRaCALA assay will facilitate rapid identification of protein-metabolite and protein-pharmaceutical interactions in a systematic and comprehensive approach.
Conflict of interest statement
Conflict of interest statement: A provisional patent has been filed by the University of Maryland.
Figures


References
-
- Lewin B. Cells. Sudbury, MA: Jones and Bartlett Publishers; 2007. p. xix.
-
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–273. - PubMed
-
- Schirmer T, Jenal U. Structural and mechanistic determinants of c-di-GMP signalling. Nat Rev Microbiol. 2009;7:724–735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

