Large collective motions regulate the functional properties of glutamate transporter trimers
- PMID: 21876140
- PMCID: PMC3174670
- DOI: 10.1073/pnas.1112216108
Large collective motions regulate the functional properties of glutamate transporter trimers
Abstract
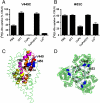
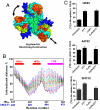
Glutamate transporters clear synaptically released glutamate to maintain precise communication between neurons and limit glutamate neurotoxicity. Although much progress has been made on the topology, structure, and function of these carriers, few studies have addressed large-scale structural motions collectively associated with substrate transport. Here we show that a series of single cysteine substitutions in the helical hairpin HP2 of excitatory amino acid transporter 1 form intersubunit disulfide cross-links within the trimer. After cross-linking, substrate uptake, but not substrate-activated anion conductance, is completely inhibited in these mutants. These disulfide bridges link residue pairs > 40 Å apart in the outward-facing crystal structure, and can be explained by concerted subunit movements predicted by the anisotropic network model (ANM). The existence of these global motions is further supported by the observation that single cysteine substitutions at the extracellular part of the transmembrane domain 8 can also be cross-linked by copper phenanthroline as predicted by the ANM. Interestingly, the transport domain in the un-cross-linked subunit of the trimer assumes an inward-facing orientation, suggesting that individual subunits potentially undergo separate transitions between outward- and inward-facing forms, rather than an all-or-none transition of the three subunits, a mechanism also supported by ANM-predicted intrinsic dynamics. These results shed light on how large collective motions contribute to the functional dynamics of glutamate transporters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

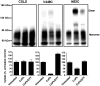
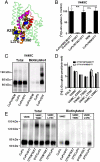


 (B). Recordings were made before (▪) and after (▴) application of 300 μM CuPh (5 min), as well as after initial treatment with 20 mM DTT (5 min, ○). The currents obtained in the absence of glutamate were subtracted from those elicited by 1 mM
(B). Recordings were made before (▪) and after (▴) application of 300 μM CuPh (5 min), as well as after initial treatment with 20 mM DTT (5 min, ○). The currents obtained in the absence of glutamate were subtracted from those elicited by 1 mM Similar articles
-
Inward-facing conformation of glutamate transporters as revealed by their inverted-topology structural repeats.Proc Natl Acad Sci U S A. 2009 Dec 8;106(49):20752-7. doi: 10.1073/pnas.0908570106. Epub 2009 Nov 19. Proc Natl Acad Sci U S A. 2009. PMID: 19926849 Free PMC article.
-
Substrate-Induced Motion between TM4 and TM7 of the Glutamate Transporter EAAT1 Revealed by Paired Cysteine Mutagenesis.Mol Pharmacol. 2019 Jan;95(1):33-42. doi: 10.1124/mol.118.113183. Epub 2018 Oct 22. Mol Pharmacol. 2019. PMID: 30348896
-
Structural rearrangements at the translocation pore of the human glutamate transporter, EAAT1.J Biol Chem. 2006 Oct 6;281(40):29788-96. doi: 10.1074/jbc.M604991200. Epub 2006 Jul 28. J Biol Chem. 2006. PMID: 16877378
-
The dual-function glutamate transporters: structure and molecular characterisation of the substrate-binding sites.Biochim Biophys Acta. 2002 Sep 10;1555(1-3):92-5. doi: 10.1016/s0005-2728(02)00260-8. Biochim Biophys Acta. 2002. PMID: 12206897 Review.
-
Slips, leaks and channels in glutamate transporters.Channels (Austin). 2008 Jan-Feb;2(1):51-8. doi: 10.4161/chan.2.1.6047. Epub 2008 Apr 2. Channels (Austin). 2008. PMID: 18690049 Review.
Cited by
-
Structure-Encoded Global Motions and Their Role in Mediating Protein-Substrate Interactions.Biophys J. 2015 Sep 15;109(6):1101-9. doi: 10.1016/j.bpj.2015.06.004. Epub 2015 Jul 2. Biophys J. 2015. PMID: 26143655 Free PMC article. Review.
-
Kinetic mechanism of Na+-coupled aspartate transport catalyzed by GltTk.Commun Biol. 2021 Jun 17;4(1):751. doi: 10.1038/s42003-021-02267-y. Commun Biol. 2021. PMID: 34140623 Free PMC article.
-
Characterizing unexpected interactions of a glutamine transporter inhibitor with members of the SLC1A transporter family.J Biol Chem. 2022 Aug;298(8):102178. doi: 10.1016/j.jbc.2022.102178. Epub 2022 Jun 23. J Biol Chem. 2022. PMID: 35752361 Free PMC article.
-
Conformational heterogeneity of the aspartate transporter Glt(Ph).Nat Struct Mol Biol. 2013 Feb;20(2):210-4. doi: 10.1038/nsmb.2471. Epub 2013 Jan 20. Nat Struct Mol Biol. 2013. PMID: 23334291
-
Discovery of (R)-N-Benzyl-2-(2,5-dioxopyrrolidin-1-yl)propanamide [(R)-AS-1], a Novel Orally Bioavailable EAAT2 Modulator with Drug-like Properties and Potent Antiseizure Activity In Vivo.J Med Chem. 2022 Sep 8;65(17):11703-11725. doi: 10.1021/acs.jmedchem.2c00534. Epub 2022 Aug 19. J Med Chem. 2022. PMID: 35984707 Free PMC article.
References
-
- Torres GE, Amara SG. Glutamate and monoamine transporters: New visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. - PubMed
-
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. - PubMed
-
- Levy LM, et al. Inducible expression of the GLT-1 glutamate transporter in a CHO cell line selected for low endogenous glutamate uptake. FEBS Lett. 1998;422:339–342. - PubMed
-
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. - PubMed
-
- Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

