Insights into Krabbe disease from structures of galactocerebrosidase
- PMID: 21876145
- PMCID: PMC3174575
- DOI: 10.1073/pnas.1105639108
Insights into Krabbe disease from structures of galactocerebrosidase
Abstract
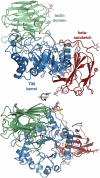
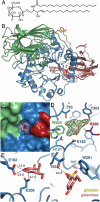
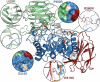
Krabbe disease is a devastating neurodegenerative disease characterized by widespread demyelination that is caused by defects in the enzyme galactocerebrosidase (GALC). Disease-causing mutations have been identified throughout the GALC gene. However, a molecular understanding of the effect of these mutations has been hampered by the lack of structural data for this enzyme. Here we present the crystal structures of GALC and the GALC-product complex, revealing a novel domain architecture with a previously uncharacterized lectin domain not observed in other hydrolases. All three domains of GALC contribute residues to the substrate-binding pocket, and disease-causing mutations are widely distributed throughout the protein. Our structures provide an essential insight into the diverse effects of pathogenic mutations on GALC function in human Krabbe variants and a compelling explanation for the severity of many mutations associated with fatal infantile disease. The localization of disease-associated mutations in the structure of GALC will facilitate identification of those patients that would be responsive to pharmacological chaperone therapies. Furthermore, our structure provides the atomic framework for the design of such drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Sneak peak at galactocerebrosidase, Krabbe disease's lysosomal hydrolase.Proc Natl Acad Sci U S A. 2011 Sep 13;108(37):15017-8. doi: 10.1073/pnas.1112653108. Epub 2011 Sep 6. Proc Natl Acad Sci U S A. 2011. PMID: 21896758 Free PMC article. No abstract available.
References
-
- Suzuki K. Globoid cell leukodystrophy (Krabbe’s disease): Update. J Child Neurol. 2003;18:595–603. - PubMed
-
- Nagara H, Ogawa H, Sato Y, Kobayashi T, Suzuki K. The twitcher mouse: Degeneration of oligodendrocytes in vitro. Brain Res. 1986;391:79–84. - PubMed
-
- Tanaka K, Nagara H, Kobayashi T, Goto I. The twitcher mouse: Accumulation of galactosylsphingosine and pathology of the sciatic nerve. Brain Res. 1988;454:340–346. - PubMed
-
- Wenger DA, Rafi MA, Luzi P, Datto J, Costantino-Ceccarini E. Krabbe disease: Genetic aspects and progress toward therapy. Mol Genet Metab. 2000;70:1–9. - PubMed
-
- Rafi MA, Luzi P, Zlotogora J, Wenger DA. Two different mutations are responsible for Krabbe disease in the Druze and Moslem Arab populations in Israel. Hum Genet. 1996;97:304–308. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

