Mapping the receptor site for alpha-scorpion toxins on a Na+ channel voltage sensor
- PMID: 21876146
- PMCID: PMC3174582
- DOI: 10.1073/pnas.1112320108
Mapping the receptor site for alpha-scorpion toxins on a Na+ channel voltage sensor
Erratum in
- Proc Natl Acad Sci U S A. 2014 Mar 4;111(9):3645
Abstract
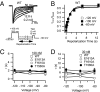
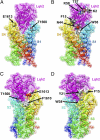
The α-scorpions toxins bind to the resting state of Na(+) channels and inhibit fast inactivation by interaction with a receptor site formed by domains I and IV. Mutants T1560A, F1610A, and E1613A in domain IV had lower affinities for Leiurus quinquestriatus hebraeus toxin II (LqhII), and mutant E1613R had ~73-fold lower affinity. Toxin dissociation was accelerated by depolarization and increased by these mutations, whereas association rates at negative membrane potentials were not changed. These results indicate that Thr1560 in the S1-S2 loop, Phe1610 in the S3 segment, and Glu1613 in the S3-S4 loop in domain IV participate in toxin binding. T393A in the SS2-S6 loop in domain I also had lower affinity for LqhII, indicating that this extracellular loop may form a secondary component of the receptor site. Analysis with the Rosetta-Membrane algorithm resulted in a model of LqhII binding to the voltage sensor in a resting state, in which amino acid residues in an extracellular cleft formed by the S1-S2 and S3-S4 loops in domain IV interact with two faces of the wedge-shaped LqhII molecule. The conserved gating charges in the S4 segment are in an inward position and form ion pairs with negatively charged amino acid residues in the S2 and S3 segments of the voltage sensor. This model defines the structure of the resting state of a voltage sensor of Na(+) channels and reveals its mode of interaction with a gating modifier toxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Mapping of scorpion toxin receptor sites at voltage-gated sodium channels.Toxicon. 2012 Sep 15;60(4):502-11. doi: 10.1016/j.toxicon.2012.03.022. Epub 2012 Apr 4. Toxicon. 2012. PMID: 22694883
-
Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3-S4 extracellular loop in domain IV of the Na+ channel alpha subunit.J Biol Chem. 1996 Jul 5;271(27):15950-62. doi: 10.1074/jbc.271.27.15950. J Biol Chem. 1996. PMID: 8663157
-
Structure-function map of the receptor site for β-scorpion toxins in domain II of voltage-gated sodium channels.J Biol Chem. 2011 Sep 23;286(38):33641-51. doi: 10.1074/jbc.M111.282509. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795675 Free PMC article.
-
Molecular mechanisms of gating and drug block of sodium channels.Novartis Found Symp. 2002;241:206-18; discussion 218-32. Novartis Found Symp. 2002. PMID: 11771647 Review.
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
Cited by
-
Voltage sensor interaction site for selective small molecule inhibitors of voltage-gated sodium channels.Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):E2724-32. doi: 10.1073/pnas.1220844110. Epub 2013 Jul 1. Proc Natl Acad Sci U S A. 2013. PMID: 23818614 Free PMC article.
-
Structural Insights into the Atomistic Mechanisms of Action of Small Molecule Inhibitors Targeting the KCa3.1 Channel Pore.Mol Pharmacol. 2017 Apr;91(4):392-402. doi: 10.1124/mol.116.108068. Epub 2017 Jan 26. Mol Pharmacol. 2017. PMID: 28126850 Free PMC article.
-
Computational methods of studying the binding of toxins from venomous animals to biological ion channels: theory and applications.Physiol Rev. 2013 Apr;93(2):767-802. doi: 10.1152/physrev.00035.2012. Physiol Rev. 2013. PMID: 23589832 Free PMC article. Review.
-
Voltage gated sodium and calcium channels: Discovery, structure, function, and Pharmacology.Channels (Austin). 2023 Dec;17(1):2281714. doi: 10.1080/19336950.2023.2281714. Epub 2023 Nov 20. Channels (Austin). 2023. PMID: 37983307 Free PMC article. Review.
-
δ-Conotoxin Structure Prediction and Analysis through Large-Scale Comparative and Deep Learning Modeling Approaches.Adv Sci (Weinh). 2024 Sep;11(35):e2404786. doi: 10.1002/advs.202404786. Epub 2024 Jul 21. Adv Sci (Weinh). 2024. PMID: 39033537 Free PMC article.
References
-
- Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. - PubMed
-
- Cestèle S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. - PubMed
-
- Catterall WA, et al. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

