Mapping the receptor site for alpha-scorpion toxins on a Na+ channel voltage sensor
- PMID: 21876146
- PMCID: PMC3174582
- DOI: 10.1073/pnas.1112320108
Mapping the receptor site for alpha-scorpion toxins on a Na+ channel voltage sensor
Erratum in
- Proc Natl Acad Sci U S A. 2014 Mar 4;111(9):3645
Abstract
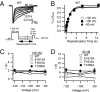
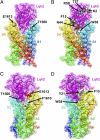
The α-scorpions toxins bind to the resting state of Na(+) channels and inhibit fast inactivation by interaction with a receptor site formed by domains I and IV. Mutants T1560A, F1610A, and E1613A in domain IV had lower affinities for Leiurus quinquestriatus hebraeus toxin II (LqhII), and mutant E1613R had ~73-fold lower affinity. Toxin dissociation was accelerated by depolarization and increased by these mutations, whereas association rates at negative membrane potentials were not changed. These results indicate that Thr1560 in the S1-S2 loop, Phe1610 in the S3 segment, and Glu1613 in the S3-S4 loop in domain IV participate in toxin binding. T393A in the SS2-S6 loop in domain I also had lower affinity for LqhII, indicating that this extracellular loop may form a secondary component of the receptor site. Analysis with the Rosetta-Membrane algorithm resulted in a model of LqhII binding to the voltage sensor in a resting state, in which amino acid residues in an extracellular cleft formed by the S1-S2 and S3-S4 loops in domain IV interact with two faces of the wedge-shaped LqhII molecule. The conserved gating charges in the S4 segment are in an inward position and form ion pairs with negatively charged amino acid residues in the S2 and S3 segments of the voltage sensor. This model defines the structure of the resting state of a voltage sensor of Na(+) channels and reveals its mode of interaction with a gating modifier toxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. - PubMed
-
- Cestèle S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. - PubMed
-
- Catterall WA, et al. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

