Forced expression of laminin beta1 in podocytes prevents nephrotic syndrome in mice lacking laminin beta2, a model for Pierson syndrome
- PMID: 21876163
- PMCID: PMC3174642
- DOI: 10.1073/pnas.1108269108
Forced expression of laminin beta1 in podocytes prevents nephrotic syndrome in mice lacking laminin beta2, a model for Pierson syndrome
Abstract
Pierson syndrome is a congenital nephrotic syndrome with ocular and neurological defects caused by mutations in LAMB2, the gene encoding the basement membrane protein laminin β2 (Lamβ2). It is the kidney glomerular basement membrane (GBM) that is defective in Pierson syndrome, as Lamβ2 is a component of laminin-521 (LM-521; α5β2γ1), the major laminin in the mature GBM. In both Pierson syndrome and the Lamb2(-/-) mouse model for this disease, laminin β1 (Lamβ1), a structurally similar homolog of Lamβ2, is marginally increased in the GBM, but it fails to fully compensate for the loss of Lamβ2, leading to the filtration barrier defects and nephrotic syndrome. Here we generated several lines of Lamβ1 transgenic mice and used them to show that podocyte-specific Lamβ1 expression in Lamb2(-/-) mice abrogates the development of nephrotic syndrome, correlating with a greatly extended lifespan. In addition, the more Lamβ1 was expressed, the less urinary albumin was excreted. Transgenic Lamβ1 expression increased the level of Lamα5 in the GBM of rescued mice, consistent with the desired increased deposition of laminin-511 (α5β1γ1) trimers. Ultrastructural analysis revealed occasional knob-like subepithelial GBM thickening but intact podocyte foot processes in aged rescued mice. These results suggest the possibility that up-regulation of LAMB1 in podocytes, should it become achievable, would likely lessen the severity of nephrotic syndrome in patients carrying LAMB2 mutations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


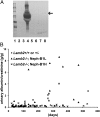
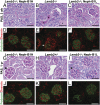

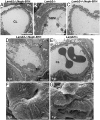
Similar articles
-
The glomerular basement membrane as a barrier to albumin.Nat Rev Nephrol. 2013 Aug;9(8):470-7. doi: 10.1038/nrneph.2013.109. Epub 2013 Jun 18. Nat Rev Nephrol. 2013. PMID: 23774818 Free PMC article. Review.
-
Laminin β2 gene missense mutation produces endoplasmic reticulum stress in podocytes.J Am Soc Nephrol. 2013 Jul;24(8):1223-33. doi: 10.1681/ASN.2012121149. Epub 2013 May 30. J Am Soc Nephrol. 2013. PMID: 23723427 Free PMC article.
-
Laminin-521 Protein Therapy for Glomerular Basement Membrane and Podocyte Abnormalities in a Model of Pierson Syndrome.J Am Soc Nephrol. 2018 May;29(5):1426-1436. doi: 10.1681/ASN.2017060690. Epub 2018 Feb 22. J Am Soc Nephrol. 2018. PMID: 29472414 Free PMC article.
-
Alport syndrome and Pierson syndrome: Diseases of the glomerular basement membrane.Matrix Biol. 2018 Oct;71-72:250-261. doi: 10.1016/j.matbio.2018.04.008. Epub 2018 Apr 16. Matrix Biol. 2018. PMID: 29673759 Free PMC article. Review.
-
Pathogenicity of a Human Laminin β2 Mutation Revealed in Models of Alport Syndrome.J Am Soc Nephrol. 2018 Mar;29(3):949-960. doi: 10.1681/ASN.2017090997. Epub 2017 Dec 20. J Am Soc Nephrol. 2018. PMID: 29263159 Free PMC article.
Cited by
-
Laminin β2 variants associated with isolated nephropathy that impact matrix regulation.JCI Insight. 2021 Mar 22;6(6):e145908. doi: 10.1172/jci.insight.145908. JCI Insight. 2021. PMID: 33749661 Free PMC article.
-
The glomerular basement membrane.Exp Cell Res. 2012 May 15;318(9):973-8. doi: 10.1016/j.yexcr.2012.02.031. Epub 2012 Mar 5. Exp Cell Res. 2012. PMID: 22410250 Free PMC article. Review.
-
Basement Membrane Defects in Genetic Kidney Diseases.Front Pediatr. 2018 Jan 29;6:11. doi: 10.3389/fped.2018.00011. eCollection 2018. Front Pediatr. 2018. PMID: 29435440 Free PMC article. Review.
-
Recent advances of animal model of focal segmental glomerulosclerosis.Clin Exp Nephrol. 2018 Aug;22(4):752-763. doi: 10.1007/s10157-018-1552-8. Epub 2018 Mar 20. Clin Exp Nephrol. 2018. PMID: 29556761 Review.
-
The glomerular basement membrane as a barrier to albumin.Nat Rev Nephrol. 2013 Aug;9(8):470-7. doi: 10.1038/nrneph.2013.109. Epub 2013 Jun 18. Nat Rev Nephrol. 2013. PMID: 23774818 Free PMC article. Review.
References
-
- Pierson M, Cordier J, Hervouuet F, Rauber G. An unusual congenital and familial congenital malformative combination involving the eye and kidney. J Genet Hum. 1963;12:184–213. - PubMed
-
- Zenker M, et al. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. - PubMed
-
- Zenker M, Pierson M, Jonveaux P, Reis A. Demonstration of two novel LAMB2 mutations in the original Pierson syndrome family reported 42 years ago. Am J Med Genet A. 2005;138:73–74. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

