Mitochondrial matrix Ca2+ as an intrinsic signal regulating mitochondrial motility in axons
- PMID: 21876166
- PMCID: PMC3174631
- DOI: 10.1073/pnas.1106862108
Mitochondrial matrix Ca2+ as an intrinsic signal regulating mitochondrial motility in axons
Abstract
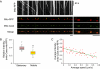
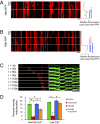
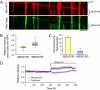
The proper distribution of mitochondria is particularly vital for neurons because of their polarized structure and high energy demand. Mitochondria in axons constantly move in response to physiological needs, but signals that regulate mitochondrial movement are not well understood. Aside from producing ATP, Ca(2+) buffering is another main function of mitochondria. Activities of many enzymes in mitochondria are also Ca(2+)-dependent, suggesting that intramitochondrial Ca(2+) concentration is important for mitochondrial functions. Here, we report that mitochondrial motility in axons is actively regulated by mitochondrial matrix Ca(2+). Ca(2+) entry through the mitochondrial Ca(2+) uniporter modulates mitochondrial transport, and mitochondrial Ca(2+) content correlates inversely with the speed of mitochondrial movement. Furthermore, the miro1 protein plays a role in Ca(2+) uptake into the mitochondria, which subsequently affects mitochondrial movement.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chang DTW, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol. 2006;80:241–268. - PubMed
-
- Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front Biosci. 1996;1:d91–d102. - PubMed
-
- Ligon LA, Steward O. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:340–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

