The former annotated human pseudogene dihydrofolate reductase-like 1 (DHFRL1) is expressed and functional
- PMID: 21876184
- PMCID: PMC3174580
- DOI: 10.1073/pnas.1103605108
The former annotated human pseudogene dihydrofolate reductase-like 1 (DHFRL1) is expressed and functional
Abstract
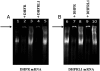
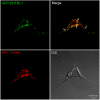
Human dihydrofolate reductase (DHFR) was previously thought to be the only enzyme capable of the reduction of dihydrofolate to tetrahydrofolate; an essential reaction necessary to ensure a continuous supply of biologically active folate. DHFR has been studied extensively from a number of perspectives because of its role in health and disease. Although the presence of a number of intronless DHFR pseudogenes has been known since the 1980s, it was assumed that none of these were expressed or functional. We show that humans do have a second dihydrofolate reductase enzyme encoded by the former pseudogene DHFRP4, located on chromosome 3. We demonstrate that the DHFRP4, or dihydrofolate reductase-like 1 (DHFRL1), gene is expressed and shares some commonalities with DHFR. Recombinant DHFRL1 can complement a DHFR-negative phenotype in bacterial and mammalian cells but has a lower specific activity than DHFR. The K(m) for NADPH is similar for both enzymes but DHFRL1 has a higher K(m) for dihydrofolate when compared to DHFR. The need for a second reductase with lowered affinity for its substrate may fulfill a specific cellular requirement. The localization of DHFRL1 to the mitochondria, as demonstrated by confocal microscopy, indicates that mitochondrial dihydrofolate reductase activity may be optimal with a lowered affinity for dihydrofolate. We also found that DHFRL1 is capable of the same translational autoregulation as DHFR by binding to its own mRNA; with each enzyme also capable of replacing the other. The identification of DHFRL1 will have implications for previous research involving DHFR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

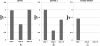
References
-
- Litwack G, editor. Vitamins and Hormones. Vol. 79. New York: Elsevier; 2008. Folic Acid and Folates. - PubMed
-
- Bertino JR. Cancer research: From folate antagonism to molecular targets. Best Pract Res Clin Haematol. 2009;22:577–582. - PubMed
-
- Bailey LN, editor. Folate in Health and Disease. New York: Marcel Dekker, Inc.; 1995. pp. 1–22.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

