German cockroach proteases and protease-activated receptor-2 regulate chemokine production and dendritic cell recruitment
- PMID: 21876326
- PMCID: PMC3250658
- DOI: 10.1159/000329132
German cockroach proteases and protease-activated receptor-2 regulate chemokine production and dendritic cell recruitment
Abstract
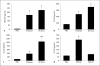
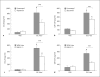
We recently showed that serine proteases in German cockroach (GC) feces (frass) decreased experimental asthma through the activation of protease-activated receptor (PAR)-2. Since dendritic cells (DCs) play an important role in the initiation of asthma, we queried the role of GC frass proteases in modulating CCL20 (chemokine C-C motif ligand 20) and granulocyte macrophage colony-stimulating factor (GM-CSF) production, factors that regulate pulmonary DCs. A single exposure to GC frass resulted in a rapid, but transient, increase in GM-CSF and a steady increase in CCL20 in the airways of mice. Instillation of protease-depleted GC frass or instillation of GC frass in PAR-2-deficient mice significantly decreased chemokine release. A specific PAR-2-activating peptide was also sufficient to induce CCL20 production. To directly assess the role of the GC frass protease in chemokine release, we enriched the protease from GC frass and confirmed that the protease was sufficient to induce both GM-CSF and CCL20 production in vivo. Primary airway epithelial cells produced both GM-CSF and CCL20 in a protease- and PAR-2-dependent manner. Finally, we show a decreased percentage of myeloid DCs in the lung following allergen exposure in PAR-2-deficient mice compared to wild-type mice. However, there was no difference in GC frass uptake. Our data indicate that, through the activation of PAR-2, allergen-derived proteases are sufficient to induce CCL20 and GM-CSF production in the airways. This leads to increased recruitment and/or differentiation of myeloid DC populations in the lungs and likely plays an important role in the initiation of allergic airway responses.
Copyright © 2011 S. Karger AG, Basel.
Figures




Similar articles
-
Protease-activated receptor 2 activation of myeloid dendritic cells regulates allergic airway inflammation.Respir Res. 2011 Sep 21;12(1):122. doi: 10.1186/1465-9921-12-122. Respir Res. 2011. PMID: 21936897 Free PMC article.
-
Mucosal sensitization to German cockroach involves protease-activated receptor-2.Respir Res. 2010 May 24;11(1):62. doi: 10.1186/1465-9921-11-62. Respir Res. 2010. PMID: 20497568 Free PMC article.
-
German cockroach frass proteases modulate the innate immune response via activation of protease-activated receptor-2.J Innate Immun. 2010;2(5):495-504. doi: 10.1159/000317195. Epub 2010 Jun 26. J Innate Immun. 2010. PMID: 20588004 Free PMC article.
-
Role of cockroach proteases in allergic disease.Curr Allergy Asthma Rep. 2012 Oct;12(5):448-55. doi: 10.1007/s11882-012-0276-1. Curr Allergy Asthma Rep. 2012. PMID: 22644866 Review.
-
Cockroach allergen exposure and risk of asthma.Allergy. 2016 Apr;71(4):463-74. doi: 10.1111/all.12827. Epub 2016 Feb 4. Allergy. 2016. PMID: 26706467 Free PMC article. Review.
Cited by
-
Tight junctions in pulmonary epithelia during lung inflammation.Pflugers Arch. 2017 Jan;469(1):135-147. doi: 10.1007/s00424-016-1917-3. Epub 2016 Dec 5. Pflugers Arch. 2017. PMID: 27921210 Free PMC article. Review.
-
Impact of Air Pollution in Airway Diseases: Role of the Epithelial Cells (Cell Models and Biomarkers).Int J Mol Sci. 2022 Mar 3;23(5):2799. doi: 10.3390/ijms23052799. Int J Mol Sci. 2022. PMID: 35269941 Free PMC article. Review.
-
Protease-activated receptor-2 activation contributes to house dust mite-induced IgE responses in mice.PLoS One. 2014 Mar 20;9(3):e91206. doi: 10.1371/journal.pone.0091206. eCollection 2014. PLoS One. 2014. PMID: 24651123 Free PMC article.
-
Chasing flies because time flies.J Innate Immun. 2015;7(1):1-2. doi: 10.1159/000368704. Epub 2014 Oct 25. J Innate Immun. 2015. PMID: 25359593 Free PMC article. No abstract available.
-
Evaluation on potential contributions of protease activated receptors related mediators in allergic inflammation.Mediators Inflamm. 2014;2014:829068. doi: 10.1155/2014/829068. Epub 2014 Apr 30. Mediators Inflamm. 2014. PMID: 24876677 Free PMC article. Review.
References
-
- Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–4578. - PubMed
-
- Chiu LL, Perng DW, Yu CH, Su SN, Chow LP. Mold allergen, Pen c 13, induced IL-8 expression in human airway epithelial cells by activated protease-activated receptor 1 and 2. J Immunol. 2007;178:5237–5244. - PubMed
-
- Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, Shin DM. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004;113:315–319. - PubMed
-
- Scarborough RM, Naughton MA, Teng W, Hung DT, Rose J, Vu TK, Wheaton VI, Turck CW, Coughlin SR. Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J Biol Chem. 1992;267:13146–13149. - PubMed
-
- Colognato R, Slupsky JR, Jendrach M, Burysek L, Syrovets T, Simmet T. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 2003;102:2645–2652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

