A history of optogenetics: the development of tools for controlling brain circuits with light
- PMID: 21876722
- PMCID: PMC3155186
- DOI: 10.3410/B3-11
A history of optogenetics: the development of tools for controlling brain circuits with light
Abstract
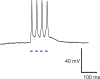
Understanding how different kinds of neuron in the brain work together to implement sensations, feelings, thoughts, and movements, and how deficits in specific kinds of neuron result in brain diseases, has long been a priority in basic and clinical neuroscience. "Optogenetic" tools are genetically encoded molecules that, when targeted to specific neurons in the brain, enable their activity to be driven or silenced by light. These molecules are microbial opsins, seven-transmembrane proteins adapted from organisms found throughout the world, which react to light by transporting ions across the lipid membranes of cells in which they are genetically expressed. These tools are enabling the causal assessment of the roles that different sets of neurons play within neural circuits, and are accordingly being used to reveal how different sets of neurons contribute to the emergent computational and behavioral functions of the brain. These tools are also being explored as components of prototype neural control prosthetics capable of correcting neural circuit computations that have gone awry in brain disorders. This review gives an account of the birth of optogenetics and discusses the technology and its applications.
Figures


References
-
- O’Neill SC, Mill JG, Eisner DA. Local activation of contraction in isolated rat ventricular myocytes. Am J Physiol. 1990;258:C1165–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

