The lens in focus: a comparison of lens development in Drosophila and vertebrates
- PMID: 21877135
- PMCID: PMC5221490
- DOI: 10.1007/s00438-011-0643-y
The lens in focus: a comparison of lens development in Drosophila and vertebrates
Abstract
The evolution of the eye has been a major subject of study dating back centuries. The advent of molecular genetics offered the surprising finding that morphologically distinct eyes rely on conserved regulatory gene networks for their formation. While many of these advances often stemmed from studies of the compound eye of the fruit fly, Drosophila melanogaster, and later translated to discoveries in vertebrate systems, studies on vertebrate lens development far outnumber those in Drosophila. This may be largely historical, since Spemann and Mangold's paradigm of tissue induction was discovered in the amphibian lens. Recent studies on lens development in Drosophila have begun to define molecular commonalities with the vertebrate lens. Here, we provide an overview of Drosophila lens development, discussing intrinsic and extrinsic factors controlling lens cell specification and differentiation. We then summarize key morphological and molecular events in vertebrate lens development, emphasizing regulatory factors and networks strongly associated with both systems. Finally, we provide a comparative analysis that highlights areas of research that would help further clarify the degree of conservation between the formation of dioptric systems in invertebrates and vertebrates.
Figures

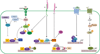




Similar articles
-
Lens crystallins of invertebrates--diversity and recruitment from detoxification enzymes and novel proteins.Eur J Biochem. 1996 Feb 1;235(3):449-65. doi: 10.1111/j.1432-1033.1996.00449.x. Eur J Biochem. 1996. PMID: 8654388 Review.
-
Analysis of the Otd-dependent transcriptome supports the evolutionary conservation of CRX/OTX/OTD functions in flies and vertebrates.Dev Biol. 2008 Mar 15;315(2):521-34. doi: 10.1016/j.ydbio.2007.12.017. Epub 2008 Jan 31. Dev Biol. 2008. PMID: 18241855 Free PMC article.
-
Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode.Development. 1997 Jan;124(1):219-31. doi: 10.1242/dev.124.1.219. Development. 1997. PMID: 9006082
-
Isolation and characterization of drosocrystallin, a lens crystallin gene of Drosophila melanogaster.Dev Biol. 1999 Mar 1;207(1):204-14. doi: 10.1006/dbio.1998.9170. Dev Biol. 1999. PMID: 10049575
-
The cellular and molecular bases of vertebrate lens regeneration.Int Rev Cytol. 2003;228:195-265. doi: 10.1016/s0074-7696(03)28005-0. Int Rev Cytol. 2003. PMID: 14667045 Review.
Cited by
-
Altered ocular parameters from circadian clock gene disruptions.PLoS One. 2019 Jun 18;14(6):e0217111. doi: 10.1371/journal.pone.0217111. eCollection 2019. PLoS One. 2019. PMID: 31211778 Free PMC article.
-
Receptor tyrosine kinases in Drosophila development.Cold Spring Harb Perspect Biol. 2013 Jun 1;5(6):a009050. doi: 10.1101/cshperspect.a009050. Cold Spring Harb Perspect Biol. 2013. PMID: 23732470 Free PMC article. Review.
-
Bi-allelic Loss-of-Function Variants in DNMBP Cause Infantile Cataracts.Am J Hum Genet. 2018 Oct 4;103(4):568-578. doi: 10.1016/j.ajhg.2018.09.004. Am J Hum Genet. 2018. PMID: 30290152 Free PMC article.
-
Jagged1 protein processing in the developing mammalian lens.Biol Open. 2019 Mar 26;8(3):bio041095. doi: 10.1242/bio.041095. Biol Open. 2019. PMID: 30890522 Free PMC article.
-
RNA-binding proteins in eye development and disease: implication of conserved RNA granule components.Wiley Interdiscip Rev RNA. 2016 Jul;7(4):527-57. doi: 10.1002/wrna.1355. Epub 2016 May 1. Wiley Interdiscip Rev RNA. 2016. PMID: 27133484 Free PMC article. Review.
References
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M. Mutations of a human homologue of the Drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet. 2000;9:363–366. - PubMed
-
- Baker NE, Yu SY. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell. 2001;104:699–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

